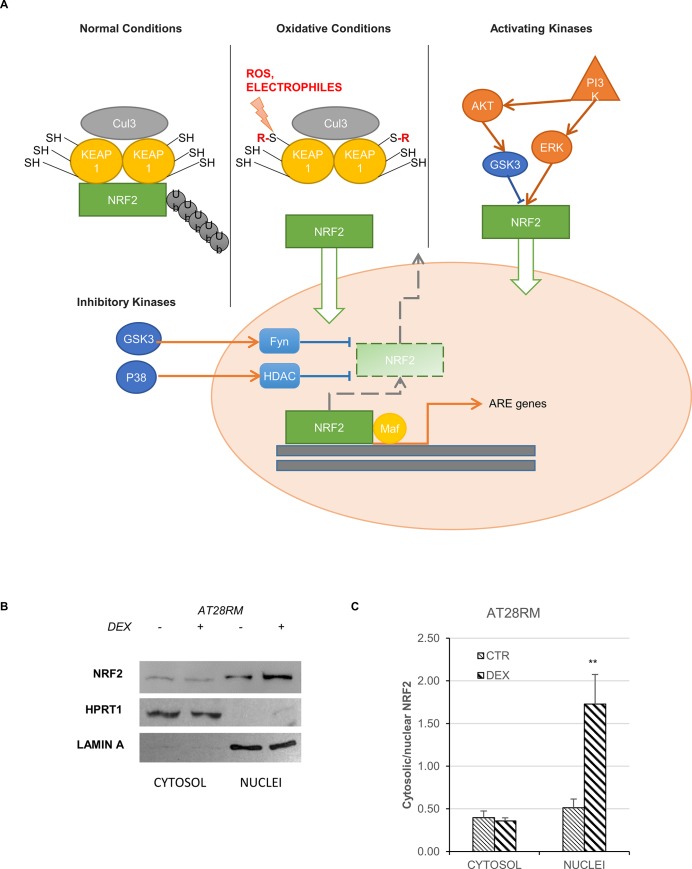
Fig 1. NRF2 activation in healthy and diseased conditions (modified from [31]) and the effect of DEX.
A) Under normal conditions NRF2 is kept at low steady state levels in the cytoplasm, a process which is tightly regulated by interaction with the Cul3 protein-adaptor KEAP1 (first branch). Under oxidative conditions, KEAP1 acts as a redox sensor releasing NRF2 and allowing its nuclear shift and the expression of target genes (second branch). In addition to the known ways of activation, phase II antioxidant enzyme induction can also be triggered by activating kinases, allowing NRF2 nuclear transport in a KEAP1-independent manner. This involves PI3K/AKT activation and/or GSK3-dependent modulation (third branch). Lastly, under chronic conditions, such as neurodegenerative diseases, the constitutive activation of inhibitory kinases leads to NRF2 detachment from ARE genes, inhibiting the antioxidant response (fourth branch). B),C) NRF2 nuclear translocation in the AT28RM cell line upon DEX treatment. B) Western blot analysis for NRF2 in the cytosolic/nuclear fractions of AT28RM cells treated with 100 nM DEX for 24 hours or not treated. HPRT1 and LAMIN A served as a loading control for the cytosolic/nuclear extracts, respectively. C) Quantification of the relative amounts of NRF2 in the cytosol and nuclei of AT28RM cells. Blot shown is representative and the histograms are the means and SEM of four independent experiments. (Wilcoxon signed rand test; *two-tailed p-values<0.05).