Figure 1.
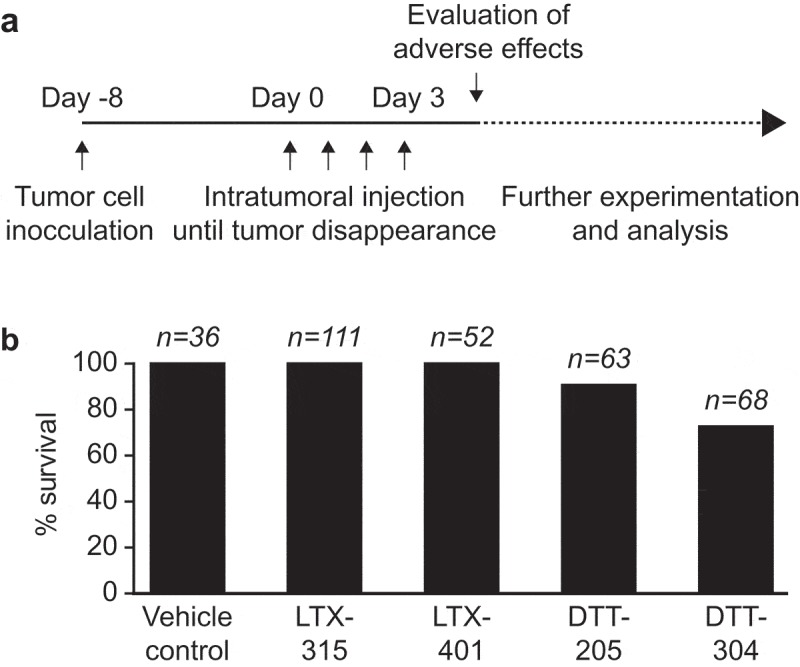
Absence of adverse toxicity in LTX-401-treated mice. In a retrospective study, the adverse toxicity of sequential intratumoral injections of oncolytic peptides and peptidomimetics at they are respective therapeutically effective dose such as LTX-315 (0.3 mg/injection), LTX-401 (0.25 mg/injection), DTT-205 and DTT-304 (both at 1.5 mg/injection) was evaluated. Note that these doses have been optimized to obtain an optimal antitumor effect. Data from in vivo experimentation utilizing oncolytic compounds in C57BL/6 mice bearing palpable subcutaneous solid tumors were evaluated and global survival was enumerated (A). The percentage of animals that survived without severe discomfort requiring their euthanasia is indicated (B).
