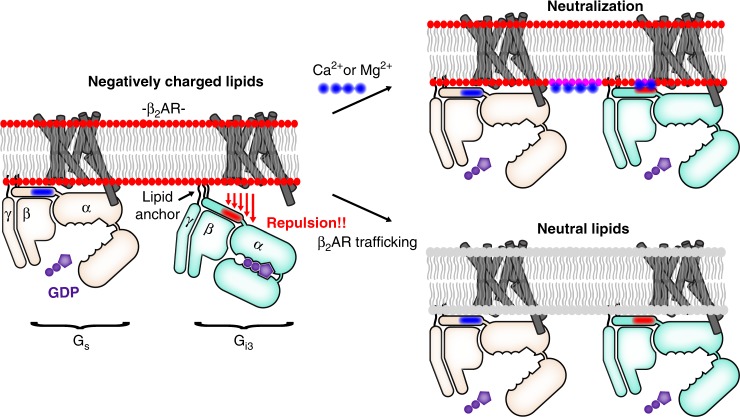
Fig. 6.
Membrane charge is a tunable modulator of β2AR−G protein interaction. Models depict epinephrine-bound β2AR. Left: In negatively charged lipids, β2AR−Gs coupling is efficient, but β2AR−Gi3 coupling is relatively inefficient, in part because β2AR−Gi3 attraction is countered by membrane-Gi3 repulsion. Specifically, negatively charged lipids repel the negatively charged EDGE motif found on the amino terminal helix of Gi3 (shown in red), a region that is positively charged in Gs (shown in blue). Right: Two mechanisms that neutralize membrane charge facilitate β2AR coupling to Gi3. These mechanisms may play a role in Gs-to-Gi switching in cardiac myocytes. Top right: Ca2+ and Mg2+ stabilize a like-charge interaction between the membrane and the EDGE motif. (Note that the effect of Ca2+ and Mg2+ may extend beyond an effect on αN positioning.) Bottom right: Epinephrine-stimulated β2AR traffics to membrane without negatively charged lipids