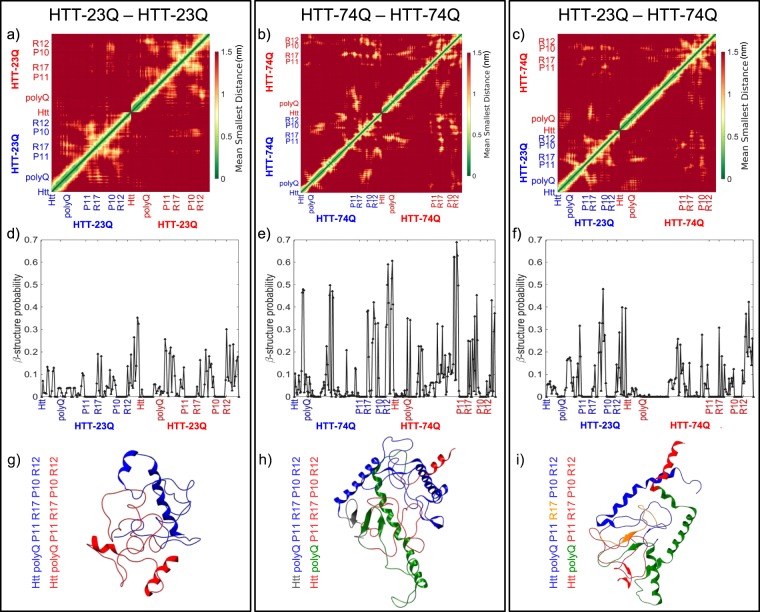
Figure 1.
Discrete molecular dynamics simulations display β structure formation indicative heterogeneous HTT dimer formation. From left to right the three panels refer to HTT-23Q–HTT-23Q, HTT-74Q–HTT-74Q and HTT-23Q–HTT-74Q systems. (a–c) The mean smallest distance maps between different residue regions. (d–f) The β probability per-residue for the different residue regions. Error bars on each point are smaller than . (g–i) Most favorable conformational structures obtained via cluster analysis. The regions of interaction between two respective proteins having β-cross conformations, the precursors of aggregation, are colored differently from the rest of the protein.