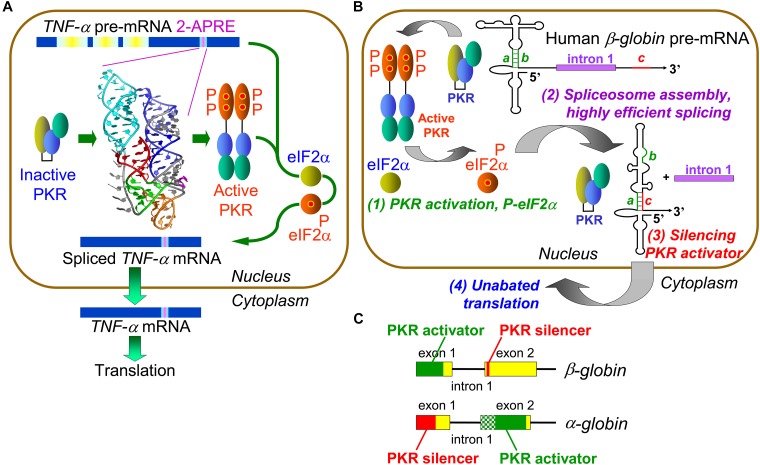
FIGURE 1.
Control of mRNA splicing by intragenic RNA activators of PKR and eIF2α phosphorylation. (A) An RNA element in TNF-α pre-mRNA controls splicing by activating PKR and thereby inducing eIF2α phosphorylation. The 104-nt TNF-α activator of PKR (2-APRE), located within the 3′-UTR, potently activates PKR through its pseudoknot structure that constrains the RNA into two parallel helices, each capable of binding a PKR monomer, facilitating PKR dimerization on RNA needed for kinase activation. Once PKR is activated, it must induce eIF-2α phosphorylation to enable efficient splicing of TNF-α mRNA. (B) Regulation of β-globin pre-mRNA splicing by an intragenic RNA activator of PKR and silencing of the PKR activator upon intron excision. (1) Within the RNA activator of PKR, consisting of 5′-terminal 124 nt within exon 1, 5 bp helix a–b (green) is critical for activity. Once PKR is activated, it must induce eIF-2α phosphorylation to enable efficient spliceosome assembly and mRNA splicing (2). Upon excision of intron 1, sequence c located near the start of exon 2 (red) displaces strand b from strand a, a rearrangement that silences the ability of mature β-globin mRNA to activate PKR (3). This renders activation of PKR transient, serving solely to promote splicing yet allowing for unimpeded synthesis of β-globin protein (4) (after Kaempfer et al., 2018). (C) Elements that activate PKR or silence PKR activation map into opposite exons in α- and β-globin pre-mRNA. The core of the α-globin RNA activator of PKR is shown in solid green within exon 2; maximal PKR activation also requires upstream RNA sequence shown in shaded green.