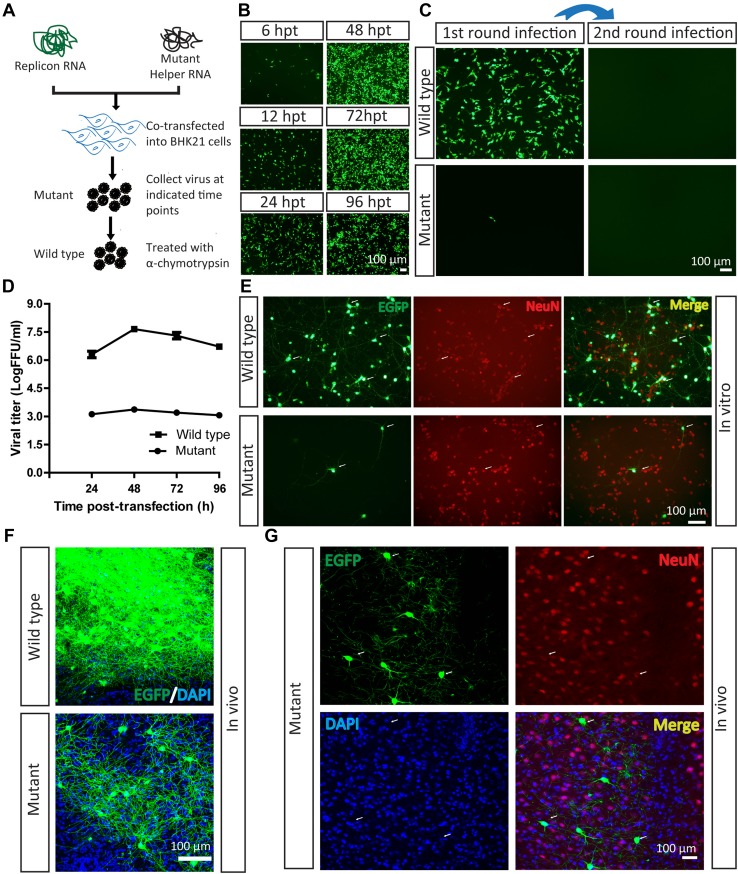
Fig. 1.
Mutant SFV VLP infects neurons in vitro and in vivo. A Work flow for preparation of SFV VLP. B Virus production. Replicon and mutant helper RNAs were co-transfected into BHK21 cells. The EGFP signals were first detected at 6 hpi, and became enhanced with time. C Mutant SFV VLP had lower entry efficiency with a single round of infection. The mutant SFV VLP was treated with α-chymotrypsin to restore infectivity and prepare the wild-type virus, then it was loaded into a BHK21 well and EGFP was observed at 24 hpi. Fresh BHK21 cells were infected with culture medium collected from cells after the first infection at 24 hpi, but no EGFP signal was detected. In addition, the mutant virus also infected BHK21 cells, and fresh BHK21 cells were infected by culture medium collected from cells after the first infection at 24 hpi, but no EGFP signal was detected. D Growth curves of the mutant and wild-type viruses. The mutant and wild-type virus (treated with α-chymotrypsin) were titered in BHK21 cells. E Wild-type and mutant SFV VLP delivered EGFP into neurons in vitro. Cultured neurons were separately infected with mutant and wild-type viruses. After 24 hpi, the EGFP signals were imaged. The neurons were stained with antibody conjugated to Cy3 against neuron-specific protein NeuN. F Wild-type (100 nL, 6 × 107 FFU/mL) and mutant viruses (100 nL, 1.2 × 104 FFU/mL) were each injected into the VPM region of mouse brain. After 24 hpi, brain sections were prepared. The wild-type virus labeled many neurons and the mutant virus labeled a few neurons. These images are representative of 15 sections (n = 3 mice). G Mutant virus was injected into the VPM region. After 24 hpi, sections were prepared. The EGFP signals co-localized with signals of the neural marker NeuN (arrows). These images are representative of 15 sections (n = 3 mice).