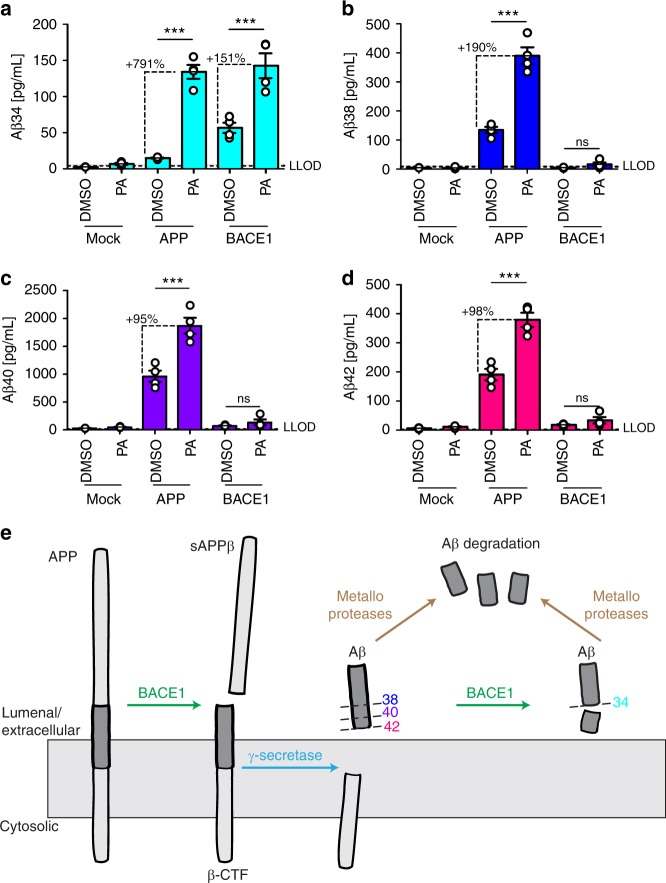
Fig. 5.
Aβ34 degradation by PA-sensitive metalloproteases. Using SH-SY5Y cells stably expressing APP695 or BACE1, MSD multiplexing to quantify the absolute amounts of Aβ34 (a), Aβ38 (b), Aβ40 (c), and Aβ42 (d) was performed. Data were collected from four independent experiments. Bars and error bars indicate mean ± s.e.m. Data were analyzed with two-way ANOVAs and significant interactions were followed up with simple main effects @treatment. ***p < 0.001, ns = nonsignificant p > 0.05. a Aβ34, interaction F(2, 18) = 23.93, p < 0.0001, simple main effects @Mock F(1, 18) = 0.12, p > 0.05, @APP F(1, 18) = 97.02, p < 0.0001, @BACE1 F(1, 18) = 50.41, p < 0.0001, b Aβ38, interaction F(2, 18) = 63.26, p < 0.0001, simple main effects @Mock F(1, 18) = 0.002, p > 0.05, @APP F(1, 18) = 197.54, p < 0.0001, @BACE1 F(1, 18) = 0.39, p > 0.05, c Aβ40, interaction F(2, 18) = 25.54, p < 0.0001, simple main effects @Mock F(1, 18) = 0.03, p > 0.05, @APP F(1, 18) = 73.97, p < 0.0001, @BACE1 F(1, 18) = 0.34, p > 0.05, d Aβ42, interaction F(2, 18) = 29.08, p < 0.0001, simple main effects @Mock F(1, 18) = 0.07, p > 0.05, @APP F(1, 18) = 97.47, p < 0.0001, @BACE1 F(1, 18) = 0.67, p > 0.05. Schematic model (e) describes the proposed APP and Aβ processing pathways involving BACE1 and metalloproteases