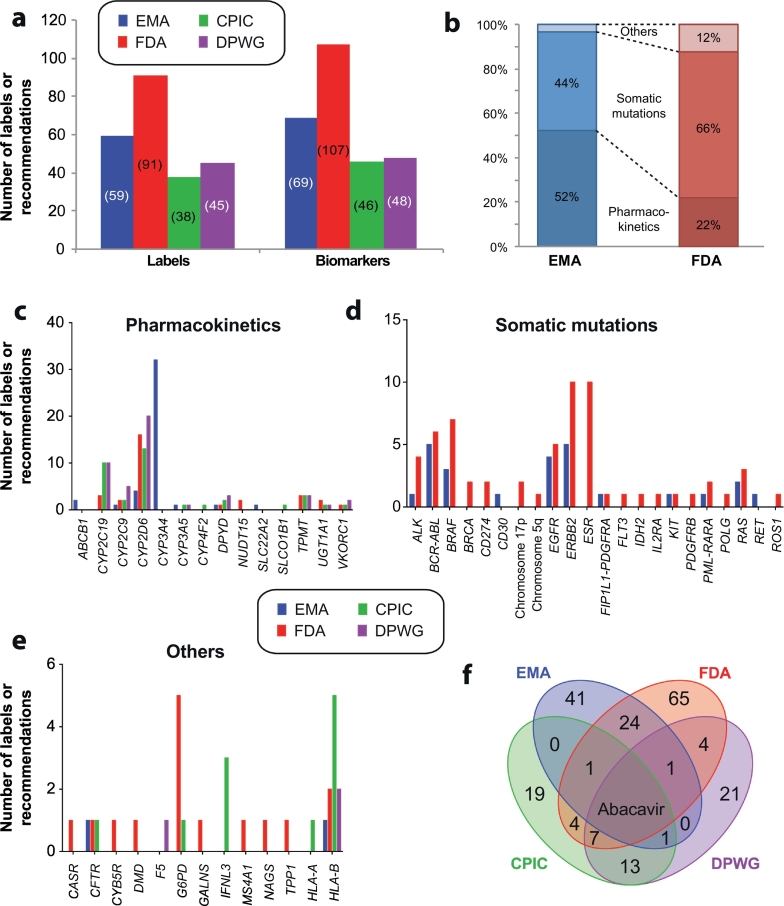
Fig. 1.
Overview of drug labels and pharmacogenetic expert guidelines. a, Overview of the number of drug labels by EMA and FDA and recommendations by CPIC and DPWG, respectively. Note that some labels and guidelines contain references to more than one biomarker. b, The majority of EMA labels refer to pharmacokinetic germline variants, whereas FDA approved labels primarily pertain to variations in the somatic genome. Only the indication, contraindication and posology sections were considered. c-e, Overview of the number of drug labels and pharmacogenetic recommendations, stratified into germline variations that impact drug pharmacokinetics (c), somatic mutations in tumors (d) and other germline variants (e). f, Venn diagram depicting the overlap of pharmacogenetic guidance from EMA (blue) and FDA (red) approved drug labels and recommendations by CPIC (green) and DPWG (purple). EMA label information was reviewed in Ehmann et al. (Ehmann et al., 2015) and only encompasses drugs registered after the foundation of EMA in 1995, which creates some lack of coherence in the comparison. FDA labels were extracted from https://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm. CPIC and DPWG guidelines were obtained from https://cpicpgx.org/guidelines and https://www.pharmgkb.org/guidelines, respectively. All sources were accessed Nov 1st 2018.