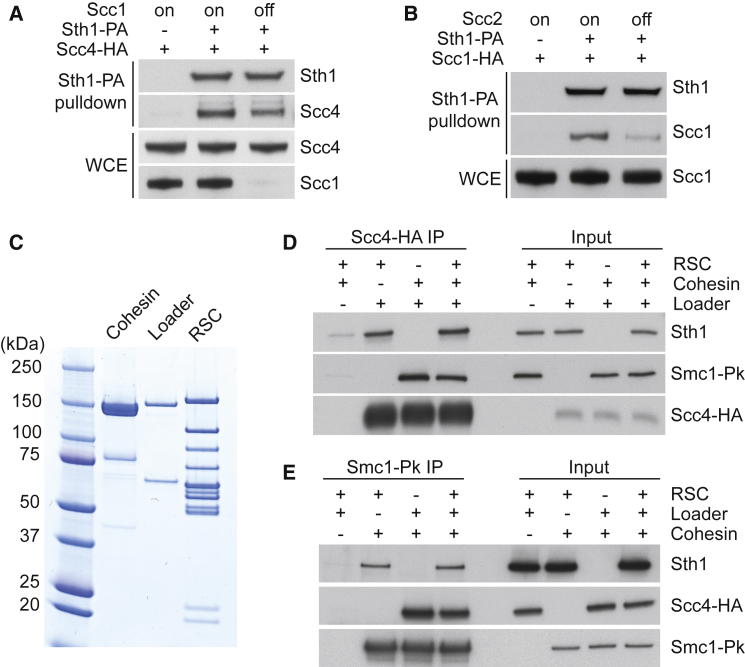
Figure 4.
RSC, Cohesin, and the Cohesin Loader Interact Directly
(A) Interaction between Sth1 and Scc4. Cells were synchronized in G1 and released into nocodazole-imposed mitotic arrest. Scc1 was depleted in one culture in G1 by methionine-induced promoter repression. Cell extracts were prepared, and protein A-tagged Sth1 was precipitated. Co-precipitation of Scc4 was analyzed by immunoblotting.
(B) Interaction between Sth1 and cohesin; as in (A), but co-precipitation of the cohesin subunit Scc1 with protein A-tagged Sth1 was evaluated by immunoblotting. Scc2 was depleted in one culture by combination of promoter repression and an auxin-inducible degron.
(C) Coomassie-stained gel showing purified cohesin, cohesin loader, and RSC chromatin remodeling complexes.
(D) RSC and the cohesin loader interact directly. Equimolar amounts of RSC, cohesin, and cohesin loader were mixed as indicated. The cohesin loader was immunoprecipitated by its hemagglutinin (HA) epitope-tagged Scc4 subunit, and the co-precipitation of RSC or cohesin was analyzed by immunoblotting.
(E) RSC and cohesin interact directly. Interaction analyses were performed as in (D), but cohesin was immunoprecipitated by its Pk epitope-tagged Smc1 subunit.
See also Figure S4 for a comparison of the Sth1-Scc4 interaction between G1 and mitotic cells, an interaction assay using Sth1K501R, and an analysis of the cohesin-cohesin loader interaction.