Figure 3.
The Ribosome-Binding Domain of NAC Exerts Chaperone Activity
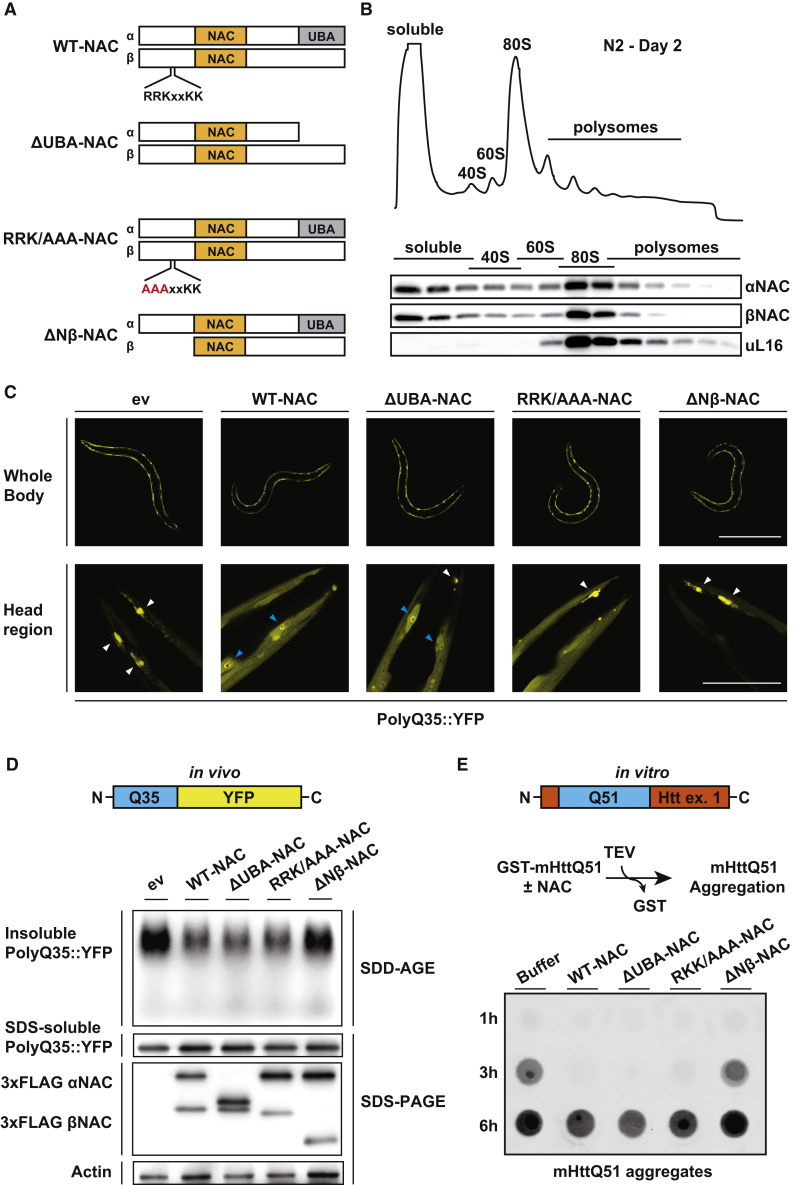
(A) Schematics showing the different heterodimeric NAC variants (α and β subunit) investigated in (C)–(E). Conserved domains (NAC and UBA) as well as the conserved ribosome-binding motif (RRKxxKK) in the β-subunit are highlighted.
(B) Sucrose density gradient analysis in wild-type N2 worms on day 2 of adulthood. Upper image shows polysome gradient profile (absorbance at 254 nm). Immunoblot images below show the distribution of NAC (α- and β-subunit) throughout the gradient. uL16 served as a ribosomal marker.
(C) Fluorescence microscope images of PolyQ35::YFP worms overexpressing WT-NAC or different mutant NAC versions shown in (A). Images were taken at day 3 of adulthood. Scalebar, 500 μm (upper row) and 50 μm (lower row). PolyQ35::YFP aggregates and cell nuclei are indicated by white and blue arrowheads, respectively. ev, empty vector.
(D) SDD-AGE immunoblot showing the PolyQ35::YFP aggregation in animals as in (C). Total levels of SDS-soluble PolyQ35::YFP and FLAG-tagged NAC variants were assessed by SDS-PAGE immunoblot analysis. Actin served as loading control.
(E) In vitro filter trap aggregation assay of mutant Huntingtin (mHttQ51) incubated with 5× molar excess of indicated NAC variants. Aggregation of GST-mHttQ51 was initiated by cleavage of the GST tag using the TEV protease. SDS-insoluble aggregates were detected with an S-tag antibody.
See also Figure S3.