Figure 4.
NAC Exerts Broad-Spectrum Chaperone Activity
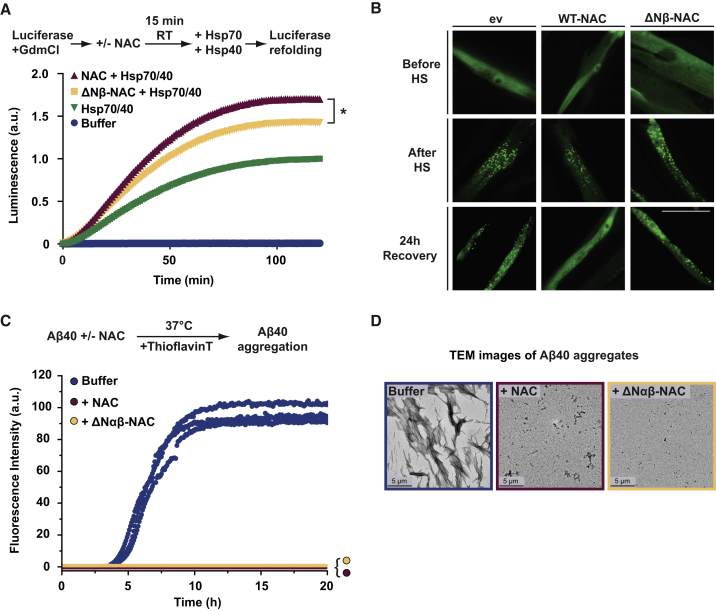
(A) In vitro chaperone refolding assays using guanidine-HCl (GdmCl)-denatured luciferase as substrate. Luciferase (0.02 μM) was preincubated with indicated NAC variants in a 1:1 molar ratio for 15 min at room temperature, and refolding was initiated by adding an Hsp70/Hsp40 chaperone system (3.2 μM/0.8 μM). Luciferase reactivation was analyzed by luminescence recording over 2 h at RT using luciferin as a substrate. Statistical significance was calculated by one-way ANOVA and Tukey post hoc test. a.u., arbitrary units. ∗p < 0.05 (n = 3).
(B) Fluorescence microscope images of C. elegans worms expressing a destabilized variant of firefly luciferase fused to EGFP (FlucDM-EGFP) and indicated NAC variants (FLAG-tagged α and βNAC) in muscle cells. Images were taken before heat shock (HS, 1 h at 33°C), directly after HS, and after 24 h recovery at 20°C. Scale bar, 20 μm. ev, empty vector.
(C) Kinetic aggregation assays of Aβ40 (32 μM, blue) incubated with an equimolar concentration of WT-NAC (red) or ΔNαβ-NAC (yellow) measured using ThioflavinT fluorescence. a.u., arbitrary units.
(D) Negative stain transmission electron micrographs of the reaction endpoint (at 20 h) for each sample shown in (C). Scale bar, 5 μm.