Figure 5.
Functional Characterization of the N-Terminal βNAC Chaperone Domain
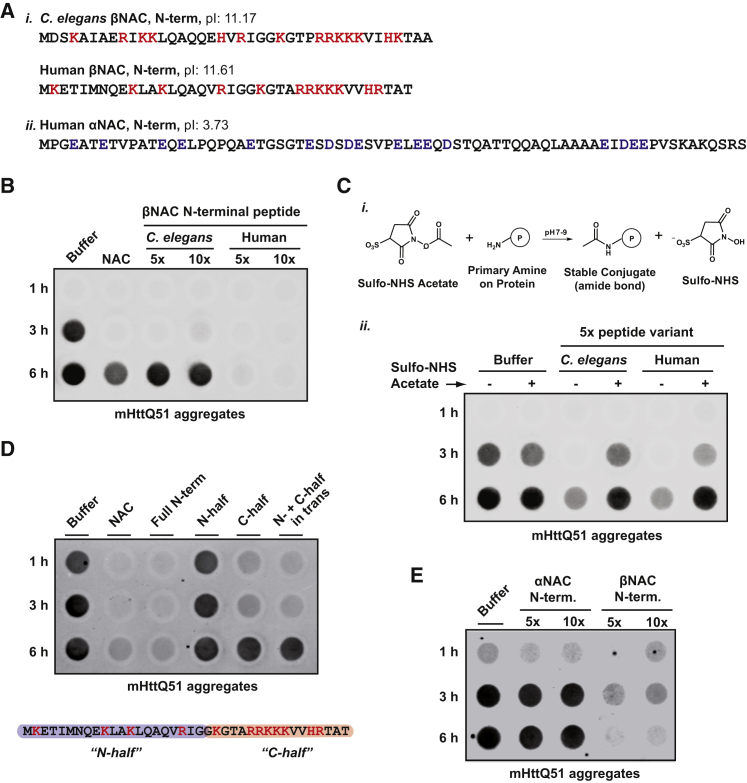
(A) (i) Peptide sequences of N-termini of βNAC from C. elegans and humans. Positively charged residues are highlighted in red. Both peptides exhibit a considerably high isoelectric point (pI). (ii) N-terminal peptide sequence of human αNAC exhibiting in contrast to βNAC peptides a low pI. Negatively charged residues are highlighted in blue.
(B) In vitro filter trap aggregation assay of mutant Huntingtin (mHttQ51) incubated with 5× or 10× molar excess of βNAC peptides shown in (A) or full-length NAC protein.
(C) (i) Chemical reaction scheme showing acylation of primary amines by Sulfo-NHS-acetate used to neutralize the positive charge in lysine residues of βNAC peptides shown in (A). (ii) In vitro filter trap aggregation assay of mutant Huntingtin (mHttQ51) incubated with 5× molar excess of peptides shown in (A) with and without Sulfo-NHS-acetate labeling.
(D) In vitro filter trap aggregation assay of mutant Huntingtin (mHttQ51) incubated with 5× molar excess of full-length NAC protein (NAC), full N-terminal βNAC peptide (Full N-term), or the N- and C-half of the peptide as indicated in the schematic below (N- and C-half highlighted in blue and red, respectively).
(E) In vitro filter trap aggregation assay of mutant Huntingtin (mHttQ51) incubated with 5× or 10× molar excess of human α- or βNAC peptides shown in (A).
See also Figure S5.