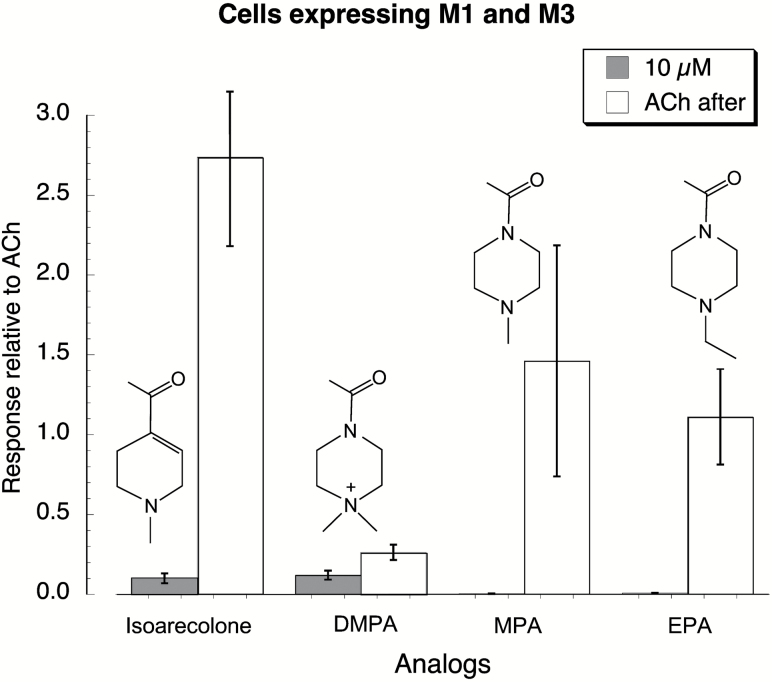
Figure 2.
Characterization of Areca alkaloids analogs on M1 and M3 acetylcholine (AChR) expressed in Xenopus oocytes. The structures of the analogs, 1-(4-methylpiperazin-1-yl) ethanone (MPA), 4-acetyl-1,1-dimethylpiperazin-1-ium (DMPA), and 1-(4-ethylpiperazin-1-yl) ethanone (EPA) are shown accompanying the corresponding data. A two-application protocol as described for Supplementary Figure S1 was used to characterize these ligands for their effects on cells expressing M1 and M3 AChR. Test compounds were applied at 10 µM, and then after a 3-min wash period 10 µM acetylcholine was applied to determine whether the initial application was able to desensitize the receptor/channel system and decrease or eliminate further responses.