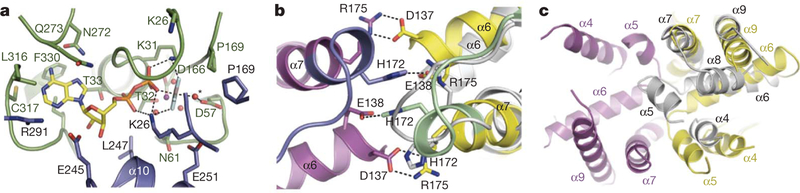
Figure 4 |. The Get3 nucleotide sensor.
a, Key interactions within the composite ATP-binding site of one subunit (green) of the closed dimer. The essential catalytic residue Asp 57 coordinates the putative nucleophilic water molecule (red sphere, asterisk), adjacent to AlF4−. The nucleotide makes further interactions with residues in the second subunit (blue), including the P-loop residue Lys 26. b, A coil-to-helix transition in the Switch II region (green and blue) is observed in the presence of ADP·AlF 4− relative to the nucleotide free state (grey subunit). Viewed ~180° from the orientation in c, looking along the dimer pseudo-two-fold axis, and coloured as in Fig. 1. Conserved, cross-monomer interactions between Switch II/α7 and the α-helical subdomain are disrupted in the open dimer (the second subunit of the nucleotide-free dimer is not visible here). c, The α-helical subdomains move apart in the open dimer (grey), and helices within each of the resulting ‘half-sites’ rearrange; helix α8, disordered in the closed dimer, inserts into the hydrophobic half-site to shield it from solvent.