Abstract
Purpose
To examine the association of a simple frailty assessment, Life Space (LS), with in-hospital mortality in elderly patients with sepsis.
Methods
We used data from a single hospital between 2014 and 2017. We included elderly patients (age ≥ 65 years) admitted to the intensive care unit (ICU) with sepsis, as defined by sepsis-3 criteria. Frailty assessment was based on a patient’s ability to independently go out of the house before the ICU admission. We termed this dichotomous score as Life Space. The primary outcome was in-hospital mortality. Logistic regression was used to investigate the association of LS with each outcome after adjusting for age, sex, and Sequential Organ Failure Assessment score.
Results
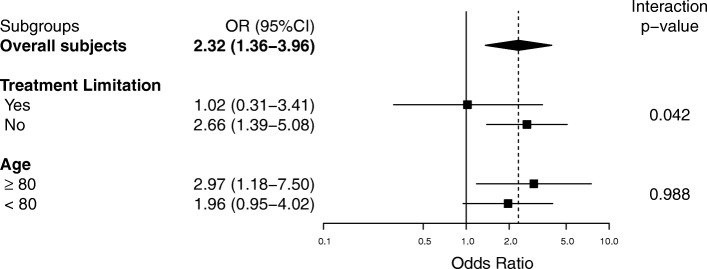
Of the 335 participants included in the final analysis, 121 (36%) were classified as frail. LS-positive patients had a higher in-hospital mortality (adjusted odds ratio (aOR) 2.32; 95% confidence interval (CI) 1.36–3.96; P = 0.002) than did LS-negative patients. We observed similar patterns in six sets of sensitivity analyses after accounting for different confounders. In subgroup analyses, significant interactions were observed in participants with versus those without treatment limitations (aOR 1.02 vs. 2.66, P for interaction = 0.042).
Conclusions
In this single-center study, frailty assessed by LS was independently associated with a higher in-hospital mortality.
Electronic supplementary material
The online version of this article (10.1186/s40560-019-0385-1) contains supplementary material, which is available to authorized users.
Keywords: Adults, Frailty, Mortality, Sepsis
Background
Sepsis is a global burden, especially in older adults, because of its high mortality and morbidity [1]. There is increasing evidence that a patient’s health status before the onset of sepsis plays a pivotal role in the progression and sequelae of sepsis [2–6]. Frailty, which is theoretically defined as a geriatric multidimensional syndrome that is assessed by one’s physiological function rather than chronological age, is one of the indices that represent a patient’s ability to recover from an episode of acute illness.
There are, however, significant barriers to the assessment of frailty in the intensive care unit (ICU). Most of the traditional scores including Frailty Index [7], Clinical Frailty Scale (CFS) [8], and Life Space Assessment [9, 10] require additional manual processes [7], which are prone to inter-rater errors and, sometimes, are time-consuming. Automated estimation of frailty was recently suggested [11]; however, it requires the implementation of administrative steps such as coding of the diagnosis, which cannot be immediately implemented in acute settings. Despite the clinical importance of bedside frailty assessment, there is a dearth of research towards the development and validation of a quick frailty assessment tool.
In this context, we focused on a simplified frailty assessment framework based on a patient’s ability to independently go out of the house and venture into the community. We termed this dichotomous score as Life Space (LS). We hypothesized a priori that LS is an independent risk factor of hospital mortality and conducted this single-center retrospective cohort study to investigate the association of LS with in-hospital mortality.
Methods
Study design
We conducted this single-center retrospective cohort study in a closed mixed-ICU system in a tertiary teaching hospital in a rural area in Japan, where the population aging rate (age ≥ 65 years) was > 30%. This study was reviewed and approved by the Kameda Medical Center’s Institutional Review Board. The committee waived the requirement for informed consent for all subjects enrolled in this study due to the retrospective design of the study. This study was conducted in accordance with the STROBE guidelines [12] for reporting.
Study population
All consecutive patients aged 65 years or older and admitted to the ICU between September 2014 and January 2017 with a diagnosis of sepsis, which was retrospectively confirmed by trained intensive care physicians using sepsis-3 criteria [13], were included. Patients who developed sepsis after the ICU admission were excluded. We excluded patients who underwent elective surgeries or stayed in the ICU for < 24 h because it was unlikely that the frailties of those patients were assessed in the ICU by physiotherapists.
Data collection
We collected the following data: age, sex, admission category (medical or emergency surgery), septic shock (defined by sepsis-3 criteria [13]), previous ICU admission, Charlson Comorbidity Index [14], treatment limitations (limitations in providing ICU-specific life-sustaining therapies such as cardiopulmonary resuscitation, mechanical ventilation, and vasopressors or renal replacement therapy), and the site of infection (abdominal, respiratory, urinary, and others). The severity of a patient’s status was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE) II score [15], Simplified Acute Physiology Score (SAPS) II [16], and Sequential Organ Failure Assessment (SOFA) score [17]. The SOFA score was manually calculated at the time of ICU admission. We also collected data regarding the use of mechanical ventilation and administration of noradrenaline and/or corticosteroids.
Frailty assessment
Frailty was initially assessed using Life Space Level (LSL). LSL is a component of the Life Space Assessment Score [10], which is widely used in the functional assessment of elderly patients [18–20]. LSL was scored by asking a patient how far he/she could move independently without limitations before the onset of the symptoms of critical illnesses, and it ranged from one’s bedroom (score = 0) to one’s town (score = 5). Physiotherapists with at least 5 years of clinical experience collected it on the first day of rehabilitation, which is usually within 24–48 h of ICU admission. If a patient was unable to provide this information, LSL was estimated on the basis of an interview with his/her family. We retrospectively collected these LSL scores from the physiotherapy electronic health records and categorized the patients into two groups: those who cannot go out of their houses (LSL = 0 or 1) were categorized in the “limited” group, while all others were categorized in the “unlimited” group (LSL ≥ 2). We named this dichotomous score as Life Space (LS) (Fig. 1).
Fig. 1.
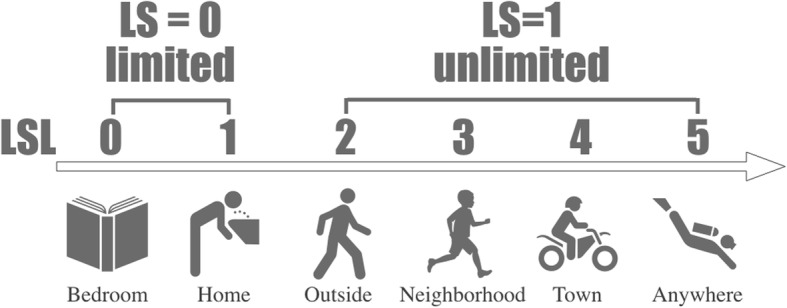
Life Space (LS). Life Space Level (LSL) was scored by asking a patient how far could he/she move independently without limitations before the onset of symptoms of critical illnesses, ranging from his/her bedroom (score = 0) to one’s town (score = 5). We retrospectively collected LSL scores and categorized patients into two groups: those who could not go out of their houses (LSL = 0 or 1) were categorized as the “limited” group (LS = 0), while all others (LSL ≥ 2) were classified as the “unlimited” group (LS = 1)
Outcome measurement
The primary outcome was in-hospital mortality. Secondary outcomes included 28-day and 90-day mortalities. All patients were followed up by the respective ICU doctors after 3 months of the ICU admission by electronic health record review.
Statistical methods
We compared the characteristics of patients in the limited and unlimited groups using the chi-square or Wilcoxon signed rank test, as appropriate. A logistic regression model adjusted for age, sex, and SOFA score on admission to the ICU was used to investigate the association between LS and each outcome. A set of potential confounders was chosen a priori based on the clinical plausibility and previous studies [4, 5, 13, 21]. LS was unlikely to be measured in severely sick patients since they were unlikely to be assessed by physiotherapists; therefore, we assumed that these study variables were missing at random [22]. We imputed the missing values using the R package “mice” [23]. A hundred datasets were created after 50 iterations for each value. Point and interval estimates were combined using Rubin’s rule [22].
Sensitivity analyses included several logistic regression models to assess the robustness of the primary analysis. The models were adjusted as follows: model 1 for age, sex, LS, SOFA score, and Charlson Comorbidity Index; model 2 for age, sex, LS, SOFA score, and admission category; model 3 for age, sex, LS, SOFA score, Charlson Comorbidity Index, and admission category; model 4 for age, sex, LS, and APACHE II score; and model 5 for age, sex, LS, and SAPS II. Next, we repeated the analyses with generalized estimating equations (GEE) in order to account for the potential clustering of cases of sepsis within each source of infection. Additionally, to assess the heterogeneity of the different levels of treatment, we conducted subgroup analysis of patients with and without treatment limitations and patients with age more than, equal to, or less than 80 years. Finally, we conducted a complete case analysis to ensure the robustness of the multiple imputations. Categorical variables were expressed as percentages, whereas continuous variables were described as means (± standard deviations (SDs)). A two-sided P value less than 0.05 was considered to indicate statistical significance. The analyses were performed using R software, version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Overall, 3103 patients were admitted to the intensive care unit within the study period; 501 patients with a diagnosis of sepsis were included in this study. Of these, 135 were excluded because their age was < 65 years, 15 were excluded because of elective surgeries, and 15 were excluded because their duration of ICU stay was < 24 h. Finally, 335 patients were included in the analyses (Fig. 2). Of these, 121 patients were categorized in the limited group based on LS. Compared with the unlimited group, the patients in the limited group were significantly older (80 vs. 77 years, P < 0.001) and had higher APACHE II score (23 vs. 21, p = 0.043), more frequent use of vasopressors (72.7% vs. 61.2%, P = 0.045) and corticosteroids (37.2% vs. 19.2%, P < 0.001), and more frequent treatment limitations (i.e., do-not-resuscitate, do-not-dialyze, and do-not-intubate) (32.2% vs. 14.5%, P < 0.001). These patients with limited function had higher in-hospital mortality (45.5% vs. 24.8%, P < 0.001), 28-day mortality (35.5% vs. 15.4%, P < 0.001), and 90-day mortality (47.9% vs. 24.8%, P < 0.001). Other patient characteristics are summarized in Table 1. Our dataset had the following missing values: LS in 79 patients, 90-day mortality in 35 patients, and 28-day mortality in 5 patients.
Fig. 2.
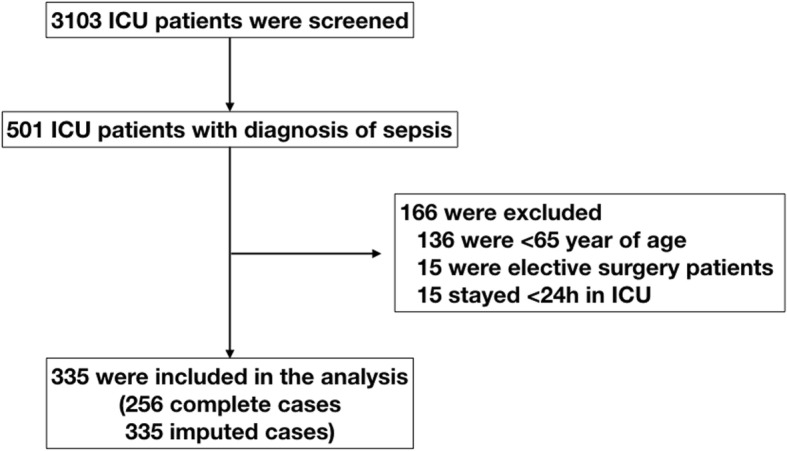
Number of patients in the intensive care unit (ICU) screened and included in primary analysis
Table 1.
Baseline characteristics of elderly patients in the intensive care unit (ICU) with sepsis
| Variable | Limited group (N = 121) | Unlimited group (N = 214) | P value | |
|---|---|---|---|---|
| Demographics | Age, years (mean [SD]) | 80 (6.90) | 77 (7.32) | < 0.001 |
| Male sex, n (%) | 75 (62.0) | 150 (70.1) | 0.162 | |
| Admission category, n (%) | 1 | |||
| Medical | 93 (76.9) | 164 (76.6) | ||
| Emergency surgery | 28 (23.1) | 50 (23.4) | ||
| Septic shock, n (%) | 81 (66.9) | 116 (54.2) | 0.031 | |
| Previous ICU admission, n (%) | 8 (6.6) | 21 (9.8) | 0.424 | |
| APACHE II score (mean [SD]) | 23 (8.79) | 21 (7.89) | 0.043 | |
| SAPS II (mean [SD]) | 53 (18.44) | 50 (16.0) | 0.094 | |
| SOFA score (mean [SD]) | 9 (3.56) | 8 (3.73) | 0.081 | |
| Charlson Comorbidity Index (mean [SD]) | 3 (2.24) | 2 (2.00) | 0.082 | |
| Treatment limitation*, n (%) | 39 (32.2) | 31 (14.5) | < 0.001 | |
| Site of infection, n (%) | 0.027 | |||
| Abdominal | 35 (28.9) | 68 (31.8) | ||
| Respiratory | 36 (29.8) | 68 (31.8) | ||
| Urinary | 30 (24.8) | 30 (14.0) | ||
| Others | 9 (7.4) | 35 (16.4) | ||
| Unknown | 11 (9.1) | 13 (6.1) | ||
| FIM (mean [SD]) | 37 (22.6) | 55 (28.8) | < 0.001 | |
| Barthel Index (mean [SD]) | 12 (19.8) | 25 (29.7) | < 0.001 | |
| Interventions | Mechanical ventilation, n (%) | 53 (43.8) | 93 (43.5) | 1 |
| Noradrenaline use, n (%) | 88 (72.7) | 131 (61.2) | 0.045 | |
| Corticosteroid use, n (%) | 45 (37.2) | 41 (19.2) | < 0.001 | |
SD standard deviation, APACHE Acute Physiology and Chronic Health Evaluation, SAPS Simplified Acute Physiology Score, SOFA Sequential Organ Failure Assessment, FIM Functional Independence Measure
*Limitation in the provision of ICU-specific life-sustaining therapies (e.g., cardiopulmonary resuscitation, mechanical ventilation, use of vasopressors, and renal replacement therapy) documented in the medical records
Association between frailty and mortality in elderly adults
Table 2 summarizes the adjusted associations between LS and each outcome. LS was associated with higher in-hospital mortality after adjustment for age, sex, and SOFA score (adjusted odds ratio (aOR) 2.32; 95% confidence interval (CI) 1.36–3.96; P = 0.002). This finding was consistent in the secondary outcomes as well; LS was an independent risk factor of 28-day mortality (aOR 3.47; 95% CI 1.87–6.46; P < 0.001) and 90-day mortality (aOR 2.56; 95% CI 1.46–4.47; P = 0.002).
Table 2.
Multivariate analysis of Life Space with primary and secondary outcomes
| Adjusted OR (95% CI) | P value | |
|---|---|---|
| In-hospital mortality | 2.32 (1.36–3.96) | 0.002 |
| 28-day mortality | 3.47 (1.87–6.46) | < 0.001 |
| 90-day mortality | 2.56 (1.46–4.47) | 0.001 |
Adjusted for age, sex, and SOFA score
OR odds ratio, CI confidence interval, SOFA Sequential Organ Failure Assessment
Sensitivity analysis
In the sensitivity analysis, the association between LS and in-hospital mortality remained similar in various multivariate analyses (Fig. 3). In the complete case analysis, however, we found no significant associations between LS and in-hospital mortality (aOR 1.43; 95% CI 0.76–2.69; P = 0.267). In addition, supplemental analyses to assess association of LS and the study outcome were shown (Additional file 1: Table S1 and S2). In the subgroup analysis, significant interactions were observed between participants with treatment limitations (aOR 1.02; 95% CI 0.31–3.41) and those without (aOR 2.66; 95% CI 1.39–5.08) (P = 0.042). No significant interactions were observed in participants divided on the basis of age (Fig. 4).
Fig. 3.
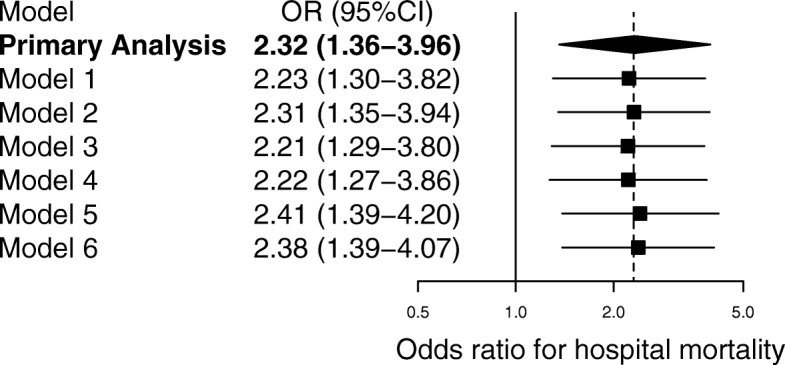
Sensitivity analyses for potential confounders. Primary analysis was further adjusted for each potential confounder. Odds ratios greater than 1.0 indicate an increased risk of death. The models were adjusted as follows: primary analysis for age, sex, and Sequential Organ Failure Assessment (SOFA) score; model 1 for age, sex, SOFA score, and Charlson Comorbidity Index; model 2 for age, sex, SOFA score, and admission category (medical or surgical); model 3 for age, sex, SOFA score, Charlson Comorbidity Index, and admission category; model 4 for age, sex, and Acute Physiology and Chronic Health Evaluation (APACHE) II score; model 5 for age, sex, and Simplified Acute Physiology Score (SAPS) II; and model 6 for age, sex, and SOFA score using generalized estimating equations (GEE) with each source of infection. Estimates are shown as mean differences with 95% confidence intervals
Fig. 4.
Adjusted odds ratios for in-hospital mortality in subgroups of patients with sepsis. In the subgroups of patients, odds ratios are indicated by solid squares. Horizontal lines represent 95% confidence intervals (95% CI). Odds ratios greater than 1.0 indicate an increased risk of in-hospital death
Discussion
In this retrospective study, we investigated 335 elderly ICU patients with sepsis to investigate the association between in-hospital mortality and LS. Multivariate analysis identified LS as an independent risk factor of in-hospital mortality. This association was consistent in multiple sensitivity analyses with different statistical assumptions. To the best of our knowledge, this is the first investigation to develop and evaluate such a straightforward assessment of frailty in patients with sepsis in ICU settings.
Recently, there has been a growing understanding that frailty may be a more robust predictor of vulnerability than chronological age alone [24]. Several studies have reported that frailty is associated with both poor short-term [2, 5, 25–27] and long-term [3, 4, 28–30] outcomes in patients in the ICU. Therefore, several statements support the use of frailty in the triage [31–36], as an entrance to the ICU, while making decisions with respect to treatment limitations [37], as an exit from the ICU. Though there is a growing demand for bedside assessment of frailty [24], only a few scores have been developed for such acute care settings. Even the most widely investigated scores, such as Frailty Index [7] and CFS [8], require additional manual steps and training, which could be substantial hurdles against their implementation in acute settings. In this context, several studies have investigated the utility of quick bedside assessments of frailty such as handgrip strength (HGS) [38], mid-arm circumference [39], and quadriceps muscle thickness [40]. Of these, HGS is one of the most widely investigated assessment tools. One multicenter prospective cohort study reported a strong positive association between HGS and in-hospital mortality in intubated patients in the ICU [38]. However, HGS is different from other frailty assessment tools in that it measures the physiological weakness during the ICU admission rather than that before the onset of critical illness. Additionally, there is no standardized protocol for the measurement of HGS, which obstructs the integration of multiple evidences into practice [41]. Overall, we are yet to find the ideal bedside assessment test of frailty in the ICU.
The advantages of LS are its simplicity and objectivity, which are essential in clinical use. Additionally, consistent with the results of the aforementioned trials with other frailty assessment tools [2, 5, 25–27, 38], LS was an independent risk factor of in-hospital mortality in our analysis. Of note, we also found that the association between frailty and mortality was influenced by the presence of treatment limitations, i.e., patients with treatment limitations had high aOR of death regardless of their frailty status. We can posit that this interaction might be a result of the different treatment approaches; however, our data and analyses were not conclusive enough to prove it. Further studies are needed to validate our findings.
Limitations
Our study had several potential limitations. First, LS data were not available in approximately a quarter of our patients. An interview with the physiotherapists involved in this study revealed that LS was missing in the following three types of patients: (1) critically unstable patients without rehabilitation orders, (2) transferred patients without rehabilitation orders, and (3) unconscious or agitated patients without family members available. This observation supports a systematic relationship between the propensity of missing values and the observed severity data (missing at random); therefore, we supported the results of our first imputation using mice rather than the results of complete case analysis, which assumes no relationship between the missing data and observed data (missing completely at random). Our results were not robust enough to conclusively affirm our hypothesis; however, they were persuasive enough to facilitate further investigation into the prospective assessment of LS.
Second, the inter-rater reliability of LS was not completely confirmed. Theoretically, an inter-rater error is possible in our dataset because we did not comprehensively define LS when the physiotherapists collected the data. Clinically, however, the probability of such an error is small. Whether one can individually go out of the house is a simple and objective question. We decided a priori to avoid using the six categories of functional assessment of LS for the sake of better inter-rater reliability. These approaches minimized the likelihood of such an error.
Third, the results of this single-center study could be prone to information bias, i.e., an unblinded association between the index test (LS) and the study outcome. However, LS was measured within 48 h of ICU admission, independent of the outcome measurement; therefore, any interaction between LS and the outcomes was minimized.
Forth, we could not directly compare LS with other frailty measurements such as Frailty Index and CFS.
Finally, we could not measure the long-term outcomes in patients with limited function. Recent studies [28, 29] have reported a strong correlation between pre-hospital frailty and long-term outcomes such as cognitive function and mortality. LS should also be assessed in this context in future trials.
Conclusion
In this single-center study that included 335 elderly adults with sepsis, LS was associated with in-hospital mortality. This association persisted across the sensitivity analyses with multiple statistical assumptions. Our findings can be utilized in the development of a quick frailty assessment tool.
Additional file
Table S1. Prediction ability of the reference and LS models for in-hospital mortality. Table S2 Propensity-match analysis* of Life Space with primary and secondary outcomes. (DOCX 15 kb)
Acknowledgements
We would like to thank all our colleagues in the intensive care unit of Kameda Medical Center for the data collection. We would also like to thank Dr. Kazuki Yoshida and Dr. Gibo Koichiro for their guidance regarding the statistic inputs.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. RU is supported by the Masason Foundation (MF) and has received a grant from MF. MF has not contributed to the study design, collection, management, analysis, and interpretation of data; the manuscript preparation; or the decision to submit the report for publication.
Availability of data and materials
The data supporting the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
- APACHE II score
Acute Physiology and Chronic Health Evaluation II score
- CFS
Clinical Frailty Scale
- CI
Confidence interval
- GEE
Generalized estimating equations
- HGS
Hand grip strength
- ICU
Intensive care unit
- LS
Life Space
- LSL
Life Space Level
- OR
Odds ratio
- SAPS II
Simplified Acute Physiology Score II
- SD
Standard deviations
- SOFA
Sequential Organ Failure Assessment
Authors’ contributions
RU has complete access to the data in the study and takes responsibility for the integrity of the data. RU, AS, and RY contributed to the study concept and design. RU, RY, and SK contributed to the acquisition of data. RU, AS, RY contributed to the analysis and interpretation of data. RU drafted the manuscript. AS, RY, SK, and YH are responsible for the critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was reviewed and approved by the Kameda Medical Center’s Institutional Review Board. The committee waived the requirement for informed consent due to the retrospective design of the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ryo Ueno, Email: rueno-tky@umin.ac.jp.
Atsushi Shiraishi, Phone: +81 4-7092-2211, Email: siris.accm@tmd.ac.jp.
Ryohei Yamamoto, Email: yamamoto.ryohei@kameda.jp.
Seibi Kobara, Email: seibi.k.pt@gmail.com.
Yoshiro Hayashi, Email: hayashi.yoshiro@kameda.jp.
References
- 1.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.CCM.0000194535.82812.BA. [DOI] [PubMed] [Google Scholar]
- 2.Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, et al. The impact of frailty on intensive care unit outcomes : a systematic review and meta-analysis. Intensive Care Med. 2017;43:1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. Can Med Assoc J. 2014;186:E95–102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 5.Flaatten H, de Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years) Intensive Care Med. 2017;43:1–9. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 6.Shears M, Takaoka A, Rochwerg B, Bagshaw SM, Johnstone J, Holding A, et al. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care. 2018;45:197–203. doi: 10.1016/j.jcrc.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Singh I, Gallacher J, Davis K, Johansen A, Eeles E, Hubbard RE. Predictors of adverse outcomes on an acute geriatric rehabilitation ward. Age Ageing. 2012;41:242–246. doi: 10.1093/ageing/afr179. [DOI] [PubMed] [Google Scholar]
- 8.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner EV, Allman RM. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Phys Ther. 2005;85:1008–1119. [PubMed] [Google Scholar]
- 10.Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51:1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a frailty risk score focusing on older people in acute care settings using electronic hospital records. Lancet. 2017;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 13.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/s001340050156. [DOI] [PubMed] [Google Scholar]
- 18.Curcio C-L, Alvarado BE, Gomez F, Guerra R, Guralnik J, Zunzunegui MV. Life-Space Assessment scale to assess mobility: validation in Latin American older women and men. Aging Clin Exp Res. 2013;25:553–560. doi: 10.1007/s40520-013-0121-y. [DOI] [PubMed] [Google Scholar]
- 19.Portegijs E, Rantakokko M, Viljanen A, Sipilä S, Rantanen T. Identification of older people at risk of ADL disability using the life-space assessment: a longitudinal cohort study. J Am Med Dir Assoc. 2016;17:410–414. doi: 10.1016/j.jamda.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y-N, Kim B-R, Uhm KE, Kim SJ, Lee S, Oh-Park M, et al. Life space assessment in stroke patients. Ann Rehabil Med. 2017;41:761. doi: 10.5535/arm.2017.41.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016;20:1–10. doi: 10.1186/s13054-016-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken: Wiley; 1987. [Google Scholar]
- 23.van BS, Groothuis-Oudshoorn K. Mice : multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 24.Montgomery C, Bagshaw SM. Frailty in the age of VIPs (very old intensive care patients) Intensive Care Med. 2017;43:1887–1888. doi: 10.1007/s00134-017-4974-y. [DOI] [PubMed] [Google Scholar]
- 25.Papageorgiou D, Gika E, Kosenai K, Tsironas K, Avramopoulou L, Sela E, et al. Frailty in elderly ICU patients in Greece: a prospective, observational study. Ann Transl Med. 2018;6:111. doi: 10.21037/atm.2018.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang L, Zhang S-Y, Zhu W-L, Pang H-Y, Zhang L, Zhu M-L, et al. Is frailty associated with short-term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol. 2015;12:662–667. doi: 10.11909/j.issn.1671-5411.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewitt J, Moug SJ, Middleton M, Chakrabarti M, Stechman MJ, McCarthy K, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254–259. doi: 10.1016/j.amjsurg.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The association of frailty with post-ICU disability, nursing home admission, and mortality. Chest. 2018;153:1378–1386. doi: 10.1016/j.chest.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietiläinen Laura, Hästbacka Johanna, Bäcklund Minna, Parviainen Ilkka, Pettilä Ville, Reinikainen Matti. Premorbid functional status as a predictor of 1-year mortality and functional status in intensive care patients aged 80 years or older. Intensive Care Medicine. 2018;44(8):1221–1229. doi: 10.1007/s00134-018-5273-y. [DOI] [PubMed] [Google Scholar]
- 30.Hope AA, Gong MN, Guerra C, Wunsch H. Frailty before critical illness and mortality for elderly medicare beneficiaries. J Am Geriatr Soc. 2015;63:1121–1128. doi: 10.1111/jgs.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guidet B, Leblanc G, Simon T, Woimant M, Quenot J-P, Ganansia O, et al. Effect of systematic intensive care unit triage on long-term mortality among critically ill elderly patients in France. JAMA. 2017;318:1450. doi: 10.1001/jama.2017.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen Y-L, Angus DC, Boumendil A, Guidet B. The challenge of admitting the very elderly to intensive care. Ann Intensive Care. 2011;1:29. doi: 10.1186/2110-5820-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angus DC. Admitting elderly patients to the intensive care unit—is it the right decision? Jama. 2017;318:1443. doi: 10.1001/jama.2017.14535. [DOI] [PubMed] [Google Scholar]
- 34.Sprung CL, Artigas A, Kesecioglu J, Pezzi A, Wiis J, Pirracchio R, et al. The Eldicus prospective, observational study of triage decision making in European intensive care units. Part II. Crit Care Med. 2012;40:132–138. doi: 10.1097/CCM.0b013e318232d6b0. [DOI] [PubMed] [Google Scholar]
- 35.Leblanc G, Boumendil A, Guidet B. Ten things to know about critically ill elderly patients. Intensive Care Med. 2017;43:217–219. doi: 10.1007/s00134-016-4477-2. [DOI] [PubMed] [Google Scholar]
- 36.Sprung CL, Danis M, Iapichino G, Artigas A, Kesecioglu J, Moreno R, et al. Triage of intensive care patients: identifying agreement and controversy. Intensive Care Med. 2013;39:1916–1924. doi: 10.1007/s00134-013-3033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guidet Bertrand, Flaatten Hans, Boumendil Ariane, Morandi Alessandro, Andersen Finn H., Artigas Antonio, Bertolini Guido, Cecconi Maurizio, Christensen Steffen, Faraldi Loredana, Fjølner Jesper, Jung Christian, Marsh Brian, Moreno Rui, Oeyen Sandra, Öhman Christina Agwald, Pinto Bernardo Bollen, Soliman Ivo W., Szczeklik Wojciech, Valentin Andreas, Watson Ximena, Zafeiridis Tilemachos, De Lange Dylan W. Withholding or withdrawing of life-sustaining therapy in older adults (≥ 80 years) admitted to the intensive care unit. Intensive Care Medicine. 2018;44(7):1027–1038. doi: 10.1007/s00134-018-5196-7. [DOI] [PubMed] [Google Scholar]
- 38.Ali NA, O’Brien JM, Hoffmann SP, Phillips G, Garland A, Finley JCW, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 39.Ma HM, Yu RHY, Woo J. Recurrent hospitalisation with pneumonia is associated with higher 1-year mortality in frail older people. Intern Med J. 2013;43:1210–1215. doi: 10.1111/imj.12258. [DOI] [PubMed] [Google Scholar]
- 40.Palakshappa JA, Reilly JP, Schweickert WD, Anderson BJ, Khoury V, Shashaty MG, et al. Quantitative peripheral muscle ultrasound in sepsis: muscle area superior to thickness. J Crit Care. 2018;47:324–330. doi: 10.1016/j.jcrc.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sousa-Santos AR, Amaral TF. Differences in handgrip strength protocols to identify sarcopenia and frailty - a systematic review. BMC Geriatr. 2017;17:238. doi: 10.1186/s12877-017-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prediction ability of the reference and LS models for in-hospital mortality. Table S2 Propensity-match analysis* of Life Space with primary and secondary outcomes. (DOCX 15 kb)
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author on reasonable request.