Abstract
Background: High-frequency ultrasound has facilitated in vivo measurement of murine ascending aorta, allowing aortic strain to be determined from 2-D imaging. Thoracic aortic aneurysms associated with mutations in fibrillin-1 (FBN1) display elastin fragmentation, which may affect aortic strain. In this study, we determined the relationship between elastin fragmentation and aortic circumferential strain in wild-type (WT) and fibrillin-1 hypomorphic (FBN1mgR/mgR) mice.
Methods and Results: Luminal diameter of the ascending aorta from WT and FBN1mgR/mgR mice was measured in systole and diastole. Expansion of the ascending aorta during systole in male and female WT mice was 0.21±0.02 mm (16.3%) and 0.21±0.01 mm (17.0%), respectively, while expansion in male and female FBN1mgR/mgR mice was 0.11±0.04 mm (4.9%) and 0.07±0.02 mm (4.5%), respectively. Reduced circumferential strain was observed in FBN1mgR/mgR mice compared with WT littermates. Elastin fragmentation was inversely correlated to circumferential strain (R2=0.628, P=0.004) and significantly correlated with aortic diameter (systole, R2=0.397, P=0.038; diastole, R2=0.515, P=0.013).
Conclusions: FBN1mgR/mgR mice had increased aortic diameter, reduced circumferential strain, and increased elastin fragmentation. Elastin fragmentation in FBN1mgR/mgR and their WT littermates was correlated with reduced circumferential strain.
Key Words: Circumferential strain, Elastin fragmentation, Marfan syndrome, Thoracic aortic aneurysm, Ultrasound
Thoracic aortic aneurysms (TAA) are abnormal dilations of the aorta associated with increased risk of life-threatening aortic dissection and rupture.1 Although the majority of TAA have not yet been associated with a specific mutation, TAA often occur in the context of inherited genetic disorders such as Marfan syndrome. TAA tissues from patients with Marfan syndrome display decreased aortic elasticity.2 Measurement of aortic diameter is a critical prognostic indicator because larger diameter is associated with higher risk of rupture.1
Mouse models of spontaneous TAA can be generated by systemic use of chemicals such as angiotensin II (AngII) or β-aminopropionitrile.3,4 Mouse models of heritable TAA can be generated by manipulation of genes responsible for extracellular matrix integrity or smooth muscle cell function such as fibrillin-1 (FBN1), fibulin-4, and ACTA-2.5–9 In this study we used the fibrillin-1 hypomorphic (FBN1mgR/mgR) mouse model, in which TAA develops aggressively early in life. Previous studies in these mice have indicated decreased elastin expression and increased elastin fragmentation in aneurysmal tissue.5,10–13 This elastin fragmentation may influence aortic circumferential strain. We hypothesized that vascular strain in the ascending aorta, measured on 2-D high-frequency ultrasound, correlated with elastin fragmentation. To test this hypothesis, we compared circumferential aortic strains in the FBN1mgR/mgR mice, which naturally develop elastin fragmentation, vs. sex-matched wild-type (WT) littermates.
Aortic diameter measurement is the primary endpoint for quantification of TAA severity in disease models. Previously, ascending aorta measurements in mice were limited to terminal endpoints.14,15 This included measurement of aortic elasticity and sheer strain in ex vivo tissues.10 Development of high-frequency ultrasound, in the range 40–50 MHz, has enabled resolution that distinguishes small changes of aortic dimension over sequential measurements.16 This resolution allows measurements of circumferential strain from 2-D images captured in vivo and enables investigation of the impact of elastin fragmentation on aortic strain.
This study used 2-D, trans-thoracic ascending aortic ultrasound measurements of the ascending aorta in WT mice and their FBN1mgR/mgR littermates to measure circumferential strain in vivo. When standardized, the 2-D, trans-thoracic ascending aortic ultrasound measurement correlates closely with traditional ex vivo measurement and has low interobserver variability.17 Finally, we assessed the correlation of these findings with elastin fragmentation.
Methods
Mice
Male and female WT C57BL/6J and FBN1mgR/mgR littermates were generated at the Jackson Laboratory (Bar Harbor, ME, USA) from stock 005704. Heterozygous FBN1mgR/+ male mice were bred with FBN1mgR/+ female mice to generate mice of desired genotypes. Mice were fed standard laboratory diet and water ad libitum and maintained on a 14:10-h light:dark cycle. Mice were transferred to a barrier facility at the University of Kentucky. All protocols were approved by the University of Kentucky IACUC.
Non-Invasive Blood Pressure Measurement
Systolic blood pressure (SBP) was measured by tail cuff using a Kent Scientific Coda 8 as described previously.18 All measurements were performed at the beginning of the light cycle. Briefly, mice were restrained and placed on a warming platform. Twenty cycles of blood pressure measurements were obtained for each mouse. Measurements <50 mmHg and >220 mmHg were excluded. Pulse rate <400 beats/min was excluded from calculation. SBP was measured on 3 consecutive days at the same time of day. Measurements represent the mean of 3 days.
Ultrasound Measurement
Ultrasound was carried out in WT and FBN1mgR/mgR mice at 11 weeks of age. Mice were anesthetized using isoflurane; dose was titrated at 2–3% wt/vol isoflurane with 2 L/min O2 to maintain heart rate 400–500 beats/min as monitored on concurrent 3-lead electrocardiogram. Ultrasound cine-loops were captured using a Vevo 2100 system with a MicroScan MS550 40-MHz transducer (VisualSonics, Toronto, ON, Canada). Frame rate was ≥300 frames/s, and 300 frames were stored per cine-loop. Aortic images were acquired from a modified right parasternal long axis view (1–2 ribs caudal to the right parasternal long axis view; Supplementary Figure 1). The probe was angled 45° relative to the chest to avoid sternum artefacts. Images were standardized to include visualization of 2 anatomical landmarks: the innominate artery and aortic valve (Supplementary Figure 2A). The ascending aorta was defined as the region between the sinotubular junction and the innominate artery. The largest ascending aortic diameter was measured in both systole and diastole of 3 separate cardiac cycles for each mouse. To facilitate measurements, a center line was drawn on the acquired image at the midpoint between the aortic walls. Aortic diameter (AoD) was measured at the largest diameter perpendicular to the center line of the aorta (Supplementary Figure 2B). AoD in systole (AoDs) was measured at physiologic systole: when the aorta was maximally dilated. AoD in diastole (AoDd) was measured at the end of diastole: defined as during the R wave (Supplementary Figure 2C). Aortic images were analyzed by 2 independent observers blinded to the experimental groups. Circumferential strain was calculated as previously described using Equation 1.19
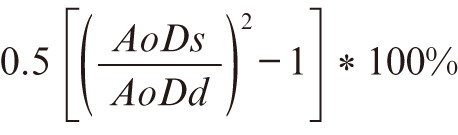 (1)
(1)
Ex Vivo Aorta Measurements
Mice were killed by overdose of ketamine:xylazine at 11 weeks of age followed by exsanguination via cardiac puncture and saline perfusion. The aorta was dissected away from surrounding tissue, and Optimal Cutting Temperature (OCT) Compound (Sakura Finetek, Torrance, CA, USA) was slowly introduced via the left ventricle to maintain aortic patency. A black plastic sheet was inserted behind the aorta and heart to increase contrast and facilitate visualization of aortic borders. The aorta was immediately imaged using a Nikon SMZ800 stereoscope and measurements were recorded using NIS-Elements AR 4.51 (Nikon Instruments, Melville, NY, USA). Fluid OCT maintained aortic patency to facilitate ex vivo and histologic analyses. Ascending aortic diameters were measured at the largest width perpendicular to the vessel.
Quantification of Elastin Fragmentation
Three proximal thoracic aortas per group were selected randomly and ≥9 tissue sections per aorta were generated using a cryostat. Tissue sections (10 µm) were generated from the aortic root to the aortic arch at 100-µm intervals. Three sections corresponding to the region of largest dilation between the sinotubular junction and the arch were analyzed. Briefly, elastin was visualized on auto-fluorescence at an excitation spectrum of 460–500 nm. Multiple high-magnification images were acquired of each section that were subsequently aggregated to a single image of the entire section using ImageJ.20 Fragmentation was defined as the presence of discernable breaks of continuous elastin fiber. The number of elastin breaks was counted by 2 independent investigators blinded to the sample identification. Individual data are represented as the mean number of elastin breaks per aortic section calculated by 2 independent investigators.
Statistical Analysis
Data are reported as mean±SEM. Statistical analysis was performed using SigmaPlot (Systat Software; San Jose, CA, USA). For 2-group comparisons, all data passed normality and equal variance tests. Paired Student’s t-test was used to compare systole and diastole from the same mouse, and unpaired Student’s t-test was used between groups. P<0.05 was considered statistically significant. Regression was calculated using Pearson’s coefficient of correlation. Bland-Altman analysis was performed by plotting the mean of aortic diameter measured ex vivo and on ultrasound vs. the difference of aortic diameter measured ex vivo and on ultrasound, with negative values indicating that ex vivo measurements are smaller than ultrasound measurements (Equation 2). Bias and limits of agreement calculated for Bland-Altman analysis were used to compare measurements of the same biological variable obtained using 2 different measurement methods.
Difference Aortic Diameter = ex vivo diameter − ultrasound diameter (2)
Results
Body Weight and SBP
The SBP and diastolic blood pressure were measured by tail cuff at 11 weeks of mice for 3 consecutive days before death at similar times of the day. Blood pressure was not significantly different in WT and FBN1mgR/mgR sex-matched littermates. In addition, body weight was not different between WT and FBN1mgR/mgR sex-matched littermates (Table). During ultrasonography, anesthesia was titrated to maintain a heart rate 400–500 beats/min. Therefore, heart rate was not significantly different between groups during ultrasonography (heart rate: WT, 453±27 beats/min; FBN1mgR/mgR, 439±26 beats/min; P=0.86). Heart rate was also not different between groups during non-invasive blood pressure measurements (Table).
Table.
Characteristics of WT and FBN1mgR/mgR Mice
| Sex / Genotype |
n | Age at ultrasound (days) |
Body weight (g) |
SBP (mmHg) |
Pulse rate (beats/min) |
AoDs (mm)† |
AoDd (mm)† |
AoDs–AoDd (mm)‡ |
|---|---|---|---|---|---|---|---|---|
| Male | ||||||||
| WT | 8 | 74.8±1.0 | 25.7±0.5 | 147±7 | 506±23 | 1.50±0.05 | 1.29±0.05*** | 0.21±0.02 |
| FBN1mgR/mgR | 8 | 74.8±1.0 | 26.2±0.8 | 139±18 | 524±31 | 2.35±0.13 | 2.24±0.15** | 0.11±0.04* |
| Female | ||||||||
| WT | 5 | 74.7±1.3 | 20.5±0.7 | 137±9 | 547±58 | 1.42±0.03 | 1.23±0.04*** | 0.21±0.01 |
| FBN1mgR/mgR | 7 | 75.2±1.2 | 20.8±0.8 | 125±13 | 443±23 | 1.64±0.07 | 1.57±0.08** | 0.07±0.02* |
Data given as mean±SEM. P>0.05 between WT vs. FBN1mgR/mgR by Student’s t-test. *P<0.05 WT vs. FBN1mgR/mgR. **P<0.01, ***P<0.001, AoDs vs. AoDd. Male and female FBN1 WT and FBN1mgR/mgR mice were aged to 11 weeks. Body weight was measured before ultrasonography. SBP and pulse rate were measured for 3 consecutive days using a tail cuff-based technique. No measurements were significantly different between WT and FBN1mgR/mgR within sex. †Male mice: WT P<0.001, FBN1mgR/mgR P=0.004; female mice: WT P<0.001, FBN1mgR/mgR P=0.009. ‡WT vs. FBN1mgR/mgR: male mice, P=0.03; female mice, P=0.01. AoDd, aortic diameter in diastole; AoDs, aortic diameter in systole; FBN1, fibrillin-1; SBP, systolic blood pressure; WT, wild type.
2-D Ultrasound Ascending Aorta Measurements
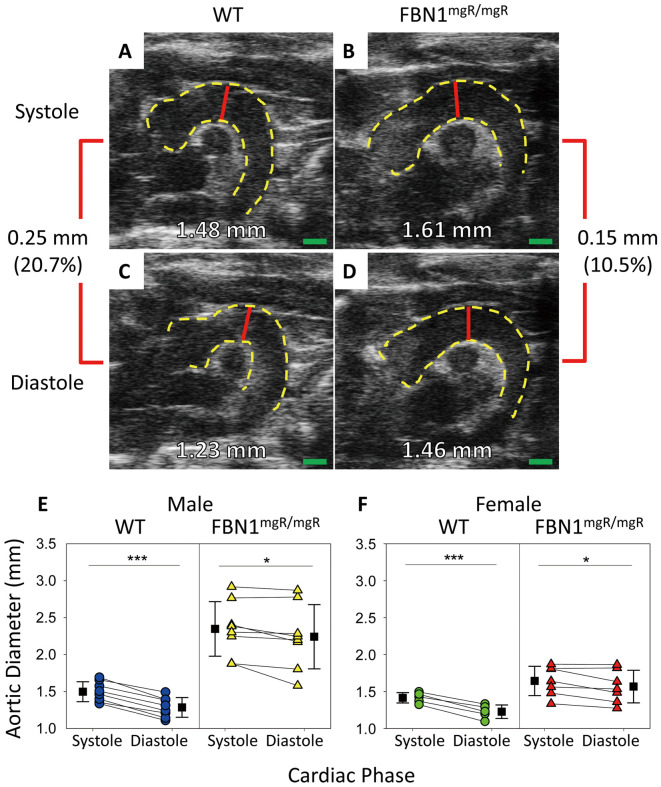
Ultrasound measurements of ascending aortas of 11-week-old male and female WT mice and their FBN1mgR/mgR littermates in systole and diastole were measured via the standardized protocol described herein (Figure 1A–D). Ascending aortic diameter measured in systole was significantly greater than that measured in diastole (Figure 1E,F) in both male and female mice (Table). The difference between systole and diastole in WT mice was larger than in FBN1mgR/mgR sex-matched littermates.
Figure 1.
Effect of cardiac cycle on ascending aortic diameters. (A–D) Representative B-mode imaging of ascending aortas from 11-week-old wild-type (WT) and fibrillin-1 (FBN1)mgR/mgR mice. (E,F) Quantification of aortic diameters in systole and diastole of (E) male and (F) female littermates. There were significant differences in aortic diameters between systole and diastole within both sex and genotype. Red line, region measured; green bar, 1 mm. Male, n=8 WT and 8 FBN1mgR/mgR; female, n=5 WT and 7 FBN1mgR/mgR; ***P<0.001, *P<0.05 between groups (paired Student’s t-test).
Circumferential Strain in Ascending Aorta During Systole
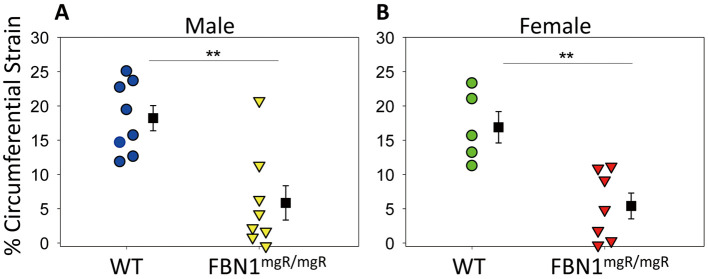
Fibrillin-1 hypomorphic mice had significantly less circumferential expansion during systole compared with their WT littermates. Green-Lagrange strain was calculated using aortic diameter on B-mode imaging during systole and diastole for each individual mouse, according to equation 1. Increased circumferential strain was observed in WT ascending aortas compared with aortas from FBN1mgR/mgR mice (Figure 2). This effect was seen in both male and female mice. For male mice: mean percent strain WT, 18.2±1.8%; FBN1mgR/mgR, 5.9±2.4% (P=0.001). For female mice: mean percent strain WT, 17.4±1.9%; FBN1mgR/mgR, 5.4±1.8% (P=0.001).
Figure 2.
Circumferential Green-Lagrange strain of the aorta during cardiac cycle in wild-type (WT) and fibrillin-1 (FBN1)mgR/mgR mice. Percent expansion was calculated by comparing aortic measurements between systole and diastole in a cardiac cycle. (A) Male and (B) female WT mice had greater percent expansion during the cardiac cycle compared with their FBN1mgR/mgR littermates. Male, n=8 WT and 8 FBN1mgR/mgR; female, n=5 WT and 7 FBN1mgR/mgR; **P<0.01 between groups (Student’s t-test).
Elastin Fragmentation: Circumferential Strain and Aortic Diameter Correlations
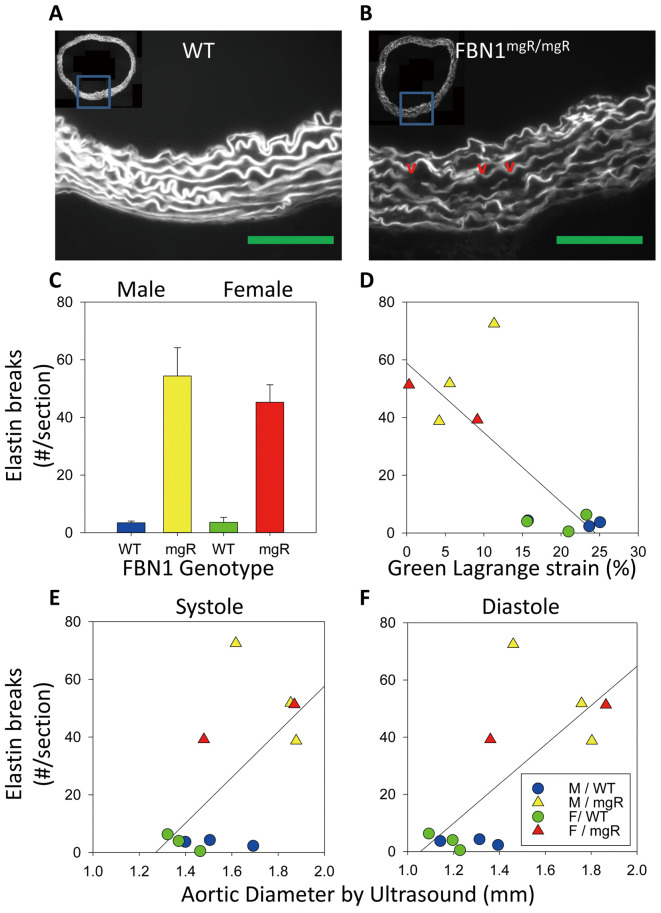
As expected, aortic sections from FBN1mgR/mgR mice had significantly greater elastin fragmentation compared with their WT counterparts (Figure 3A–C). Elastin fragmentation was also significantly inversely correlated with in vivo circumferential strain (R2=0.628, P=0.004) and positively correlated with both systolic and diastolic aortic diameters (systole, R2=0.397, P=0.038; diastole, R2=0.515, P=0.013; Figure 3D–F). On multiple linear regression modeling, we also determined that the FBN1 genotype contributed significantly to differences in elastin fragmentation (P=0.001) and that elastin fragmentation did not correlate with sex (P=0.414).
Figure 3.
Elastin fragmentation of wild-type (WT) and fibrillin-1 (FBN1)mgR/mgR correlated with ascending aortic diameter. (A,B) Representative elastin autofluorescence imaging of elastin fragmentation from WT and FBN1mgR/mgR mice at the largest ascending aortic diameter. Green line, 100 µm. (C) Elastin fragmentation quantified. (D) Elastin fragmentation vs. circumferential strain (R2=0.628, P=0.004). (E,F) Elastin breaks vs. aortic diameter in (E) systole (R2=0.397, P=0.038) and (F) diastole (R2=0.515, P=0.013). n=3, male WT, male mgR, female WT. n=2, female mgR.
Discussion
Previously, our group reported aortic diameters in aneurysm studies without specifying the cardiac phase during which these in vivo measurements were taken.16,21–23 In this study, we have demonstrated that in vivo 2-D ultrasound measurements are accurate and correlate with in situ measurements of exposed aortas (Supplementary Figure 3). Furthermore, we quantified the difference between measurements taken in systole and diastole in the thoracic aorta (Figure 1). This suggested that 2-D trans-thoracic ultrasound can be used to calculate circumferential aortic strain, despite the fact that the thoracic aorta experiences increased longitudinal displacement due to its proximity to the beating heart.19,24 Using a mouse model of elastin fragmentation, we further investigated whether differences in aortic strain between FBN1mgR/mgR mice and their WT littermates correlated with elastin fragmentation.
Previously, circumferential strain has been characterized in the AngII-induced mouse model of aortic aneurysms.25 These analyses, however, have not been performed for mouse models of heritable thoracic aortic aneurysms, such as FBN1mgR/mgR mice. FBN1mgR/mgR mice were used in this study because of the rapid development of pronounced thoracic aneurysms.5,26 Mouse models with mutations in FBN1 exhibit decreased aortic elastin expression, elastin fragmentation, and TAA.10 Aortas from WT mice experienced greater circumferential strain during systole compared with aortas from FBN1mgR/mgR mice. In this study, SBP was acquired using a standardized protocol on a system that measures the variance in tail volume in conjunction with a pressure cuff.18 We have demonstrated previously that there is good correlation between blood pressure measurements obtained using this process and those obtained using telemetry.27 Although absolute blood pressure measurements can differ between instruments, there were no differences detected in SBP between WT and FBN1mgR/mgR mice. There was also no significant difference in heart rate measured while conscious or under anesthesia. The correlation between blood pressure and circumferential strain was not significant. Blood pressures, however, were not measured during ultrasound. We cannot assume that blood pressure in the awake state is equal to blood pressure during ultrasound in the anesthetized state. Although this suggests that the increase in aortic stiffness is a function of intrinsic aortic tissue mechanics, this point merits further study. Indeed, this increase in aortic stiffness has been measured in other mouse models of spontaneous thoracic and abdominal aortic aneurysm and dissection. Circumferential aortic strain in the AngII-induced mouse model of abdominal aortic aneurysms is approximately 15% in control mice and 5% in AngII-infused mice.25 These strains are consistent when measured using speckle tracking technology.28 Interestingly, AngII-induced thoracic aortic aneurysms also had reduced circumferential strain. Circumferential strains in this model decrease from 20% before infusion to 10% after AngII infusion.29 Data from AngII-induced aortic aneurysms are in agreement with the present results in a heritable model of thoracic aortic aneurysm.
Strains have been measured ex vivo in the FBN1mgR/mgR mouse model. Previously, Lee et al demonstrated that compressive forces, measured using atomic force microscopy acting on aortas from FBN1mgR/mgR mice, produced greater deformation under stress.10 This was attributed to the loss of elasticity in FBN1mgR/mgR aortas as evidenced by reduced area fraction of elastic fibers. We quantified elastin fragmentation as a proxy of aortic elastic behavior. While elastin fragmentation was correlated with reduced circumferential strain in response to circumferential tensile forces in vivo, this analysis is limited by the assumption that loss of continuous elastin layers leads to loss of elasticity. Paradoxically, loss of elasticity can explain both the loss of resistance to compressive forces outlined by Lee et al10 and the increased resistance to circumferential tensile forces shown in the present study via the Poisson effect. Together, these observations imply that increased elastin fragmentation in FBN1mgR/mgR aortic tissue may be responsible for reduced aortic strain observed during systole in FBN1mgR/mgR mouse.
The primary purpose of this study was to determine the relationship between circumferential aortic strain measured in vivo and elastin fragmentation. We additionally quantified aortic diameter increases during systole compared with diastole in both WT and FBN1mgR/mgR mice. The significant size differences indicate that cardiac cycle influences interpretation of murine studies that report thoracic aortic diameters. Based on the present data, the excursion of the aorta in systole can be as great as 0.2 mm in WT aortas and 0.1 mm in aneurysmal aortas. This difference is larger than error attributable to interobserver variability in the present dataset, where bias between observers was 0.08±0.08 mm in systole and −0.07±0.06 mm in diastole (Supplementary Figure 4). Thus, specifying whether ascending aortic diameter was measured in either systole or diastole is especially important in order to detect small differences, ensure accuracy, and increase study power. Accounting for this phenomenon is important when ascending aortic diameter is measured across different studies. This discrepancy occurs because studies have reported aortic diameters in systole, diastole, both, or omit reporting cardiac cycle.16,29–32 In lieu of these data, a correction factor of approximately 17% in C57BL/6J WT aortas and 5% in FBN1mgR/mgR aneurysmal aortas may be taken to approximate measurements taken in systole compared with diastole.
Conclusions
In mice that spontaneously develop elastin fragmentation, reduced circumferential strain is correlated with increased elastin fragmentation, and 2-D ultrasound data can be used to measure circumferential strain in vivo.
Disclosures
The authors declare no conflicts of interest.
Supplementary Files
Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3 Supplementary Figure 4
Acknowledgments
The authors’ research work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL133723 and an American Heart Association Strategically Focus Network award number 18SFRN33900001. J.Z.C. and M.B.S. are supported by NCATS UL1TR001998. M.B.S. is also supported by the University of Kentucky College of Medicine SChoLARs Career Development Program. H.S. is supported by an AHA postdoctoral fellowship (18POST33990468). The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiology and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, Society for Vascular Medicine.. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010; 121: e266–e369. [DOI] [PubMed] [Google Scholar]
- 2. Akazawa Y, Motoki N, Tada A, Yamazaki S, Hachiya A, Matsuzaki S, et al.. Decreased aortic elasticity in children with Marfan syndrome or Loeys-Dietz syndrome. Circ J 2016; 80: 2369–2375. [DOI] [PubMed] [Google Scholar]
- 3. Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA.. Angiotensin II infusion promotes ascending aortic aneurysms: Attenuation by CCR2 deficiency in ApoE-/- mice. Clin Sci (Lond) 2010; 118: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ren W, Liu Y, Wang X, Jia L, Piao C, Lan F, et al.. Beta-aminopropionitrile monofumarate induces thoracic aortic dissection in C57Bl/6 Mice. Sci Rep 2016; 6: 28149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, et al.. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA 1999; 96: 3819–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, et al.. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 2004; 114: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, et al.. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol 2006; 26: 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Milewicz DM, Ostergaard JR, Ala-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, et al.. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A 2010; 152a: 2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Peters A, Papke CL, Villamizar C, Ringuette LJ, Cao J, et al.. Loss of smooth muscle alpha-actin leads to nf-kappab-dependent increased sensitivity to angiotensin II in smooth muscle cells and aortic enlargement. Circ Res 2017; 120: 1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JJ, Galatioto J, Rao S, Ramirez F, Costa KD.. Losartan attenuates degradation of aorta and lung tissue micromechanics in a mouse model of severe Marfan syndrome. Ann Biomed Eng 2016; 44: 2994–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong W, Meisinger T, Knispel R, Worth JM, Baxter BT.. Mmp-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ Res 2012; 110: e92–e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marque V, Kieffer P, Gayraud B, Lartaud-Idjouadiene I, Ramirez F, Atkinson J.. Aortic wall mechanics and composition in a transgenic mouse model of Marfan syndrome. Arterioscler Thromb Vasc Biol 2001; 21: 1184–1189. [DOI] [PubMed] [Google Scholar]
- 13. Howell DW, Popovic N, Metz RP, Wilson E.. Regional changes in elastic fiber organization and transforming growth factor beta signaling in aortas from a mouse model of Marfan syndrome. Cell Tissue Res 2014; 358: 807–819. [DOI] [PubMed] [Google Scholar]
- 14. Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, et al.. A murine model of thoracic aortic aneurysms. J Surg Res 2003; 115: 157–163. [DOI] [PubMed] [Google Scholar]
- 15. Ikonomidis JS, Gibson WC, Butler JE, McClister DM, Sweterlitsch SE, Thompson RP, et al.. Effects of deletion of the tissue inhibitor of matrix metalloproteinases-1 gene on the progression of murine thoracic aortic aneurysms. Circulation 2004; 110(11 Suppl 1): II268–II273. [DOI] [PubMed] [Google Scholar]
- 16. Rateri DL, Davis FM, Balakrishnan A, Howatt DA, Moorleghen JJ, O’Connor WN, et al.. Angiotensin II Induces region-specific medial disruption during evolution of ascending aortic aneurysms. Am J Pathol 2014; 184: 2586–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawada H, Chen JZ, Wright BC, Moorleghen JJ, Lu HS, Daugherty A.. Ultrasound imaging of the thoracic and abdominal aorta in mice to determine aneurysm dimensions. J Vis Exp 2019; 145: e59013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daugherty A, Rateri D, Hong L, Balakrishnan A.. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J Vis Exp 2009; 27: e1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goergen CJ, Barr KN, Huynh DT, Eastham-Anderson JR, Choi G, Hedehus M, et al.. In vivo quantification of murine aortic cyclic strain, motion, and curvature: Implications for abdominal aortic aneurysm growth. J Magn Reson Imaging 2010; 32: 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Preibisch S, Saalfeld S, Tomancak P.. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009; 25: 1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A.. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg 2006; 44: 372–376. [DOI] [PubMed] [Google Scholar]
- 22. Poduri A, Owens AP 3rd, Howatt DA, Moorleghen JJ, Balakrishnan A, Cassis LA, et al.. Regional variation in aortic AT1b receptor mRNA abundance is associated with contractility but unrelated to atherosclerosis and aortic aneurysms. PLoS One 2012; 7: e48462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rateri DL, Moorleghen JJ, Knight V, Balakrishnan A, Howatt DA, Cassis LA, et al.. Depletion of endothelial or smooth muscle cell-specific angiotensin II type 1a receptors does not influence aortic aneurysms or atherosclerosis in LDL receptor deficient mice. PLoS One 2012; 7: e51483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plonek T, Berezowski M, Kurcz J, Podgorski P, Sasiadek M, Rylski B, et al.. The evaluation of the aortic annulus displacement during cardiac cycle using magnetic resonance imaging. BMC Cardiovasc Disord 2018; 18: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trachet B, Fraga-Silva RA, Londono FJ, Swillens A, Stergiopulos N, Segers P.. Performance comparison of ultrasound-based methods to assess aortic diameter and stiffness in normal and aneurysmal mice. PLoS One 2015; 10: e0129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, et al.. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet 1997; 17: 218–222. [DOI] [PubMed] [Google Scholar]
- 27. Haggerty CM, Mattingly AC, Gong MC, Su W, Daugherty A, Fornwalt BK.. Telemetric blood pressure assessment in angiotensin II-Infused ApoE-/- Mice: 28 day natural history and comparison to tail-cuff measurements. PLoS One 2015; 10: e0130723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Favreau JT, Nguyen BT, Gao I, Yu P, Tao M, Schneiderman J, et al.. Murine ultrasound imaging for circumferential strain analyses in the angiotensin II abdominal aortic aneurysm model. J Vasc Surg 2012; 56: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trachet B, Piersigilli A, Fraga-Silva RA, Aslanidou L, Sordet-Dessimoz J, Astolfo A, et al.. Ascending aortic aneurysm in angiotensin II-infused mice: Formation, progression, and the role of focal dissections. Arterioscler Thromb Vasc Biol 2016; 36: 673–681. [DOI] [PubMed] [Google Scholar]
- 30. Lee L, Cui JZ, Cua M, Esfandiarei M, Sheng X, Chui WA, et al.. Aortic and cardiac structure and function using high-resolution echocardiography and optical coherence tomography in a mouse model of Marfan syndrome. PLoS One 2016; 11: e0164778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shen M, Lee J, Basu R, Sakamuri SS, Wang X, Fan D, et al.. Divergent roles of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler Thromb Vasc Biol 2015; 35: 888–898. [DOI] [PubMed] [Google Scholar]
- 32. Jiao Y, Li G, Li Q, Ali R, Qin L, Li W, et al.. MTOR (mechanistic target of rapamycin) inhibition decreases mechanosignaling, collagen accumulation, and stiffening of the thoracic aorta in elastin-deficient mice. Arterioscler Thromb Vasc Biol 2017; 37: 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3 Supplementary Figure 4