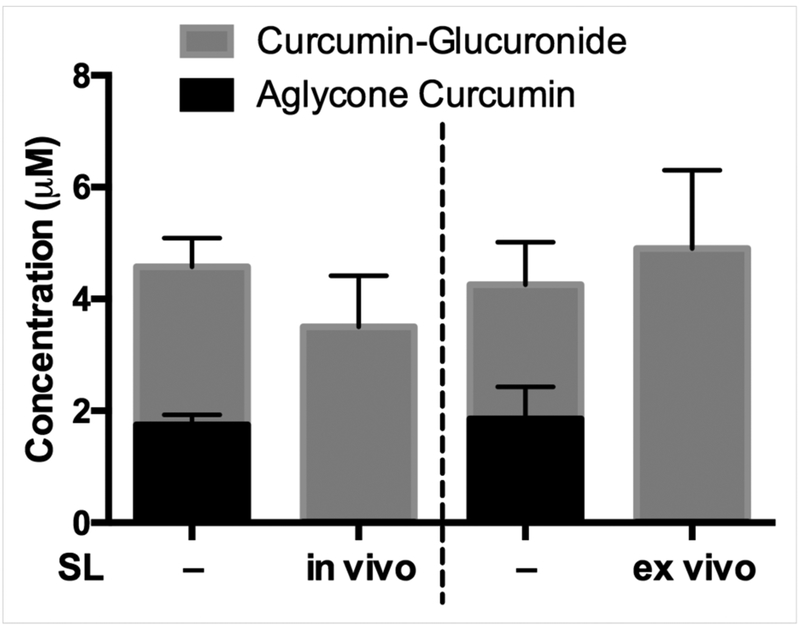
Figure 3. Capacity of marrow to hydrolyze curcumin-glucuronide distributing to bone following oral administration of curcumin.
Bone marrow harvested 30 min after in vivo treatment of female, 4 wk C57BL/6J mice with curcumin (500 mg/kg by oral gavage) was resuspended in sodium acetate buffer as described for assay of aglycone and glucuronidated curcumin. For in vivo saccharolactone (SL) treatments, which did not alter serum aglycone curcumin levels (data not shown), SL (1 g/kg vs. normal saline) was administered via intraperitoneal injection 1 hour prior to curcumin treatment (left 2 bars). For ex vivo SL experiments, bone marrow from curcumin-treated mice was resuspended in sodium acetate buffer with or without 10 mM SL (right 2 bars). Data expressed as mean ± SEM (n=3–4/group).