Abstract
Intervertebral disc degeneration (IDD) is the major cause of low back pain which incurs a significant public‐health and economic burden. The aetiology of IDD is complex, with developmental, genetic, biomechanical and biochemical factors contributing to the disease development. Deregulated phenotypes of nucleus pulposus cells, including aberrant differentiation, apoptosis, proliferation and extracellular matrix deposition, are involved in the initiation and progression of IDD. Non‐coding RNAs, including long non‐coding RNAs (lncRNAs), have recently been identified as important regulators of gene expression. Research into their roles in IDD has been very active over the past 5 years. Our review summarizes current research regarding the roles of deregulated lncRNAs (eg, RP11‐296A18.3, TUG1, HCG18) in modulating nucleus pulposus cell functions in IDD. These exciting findings suggest that specific modulation of lncRNAs or their downstream signalling pathways might be an attractive approach for developing novel therapeutics for IDD.
Keywords: cell death, extracellular matrix, low back pain, pathogenesis, prolapsed disc
1. INTRODUCTION
Low back pain is a common but debilitating disorder, creating a huge public‐health and economic burden. Enormous efforts have been put forth to investigate the pathogenesis and optimise the clinical management of low back pain.1, 2, 3, 4 The aetiology of low back pain is manifold, among which intervertebral disc degeneration (IDD) is a major but poorly understood contributing factor.5, 6, 7 The pathogenesis of IDD can be attributed to a myriad of risk factors, including lifestyles (eg, smoking, alcohol consumption, occupation), genetic predisposition, and ageing.8, 9, 10, 11, 12 However, the precise cellular and molecular mechanisms linking these factors to IDD development still remain unclear. In this respect, a growing body of evidence has suggested that nucleus pulposus (NP) cells are crucial for preserving the integrity of intervertebral discs (IVD) via their roles in producing extracellular matrix (ECM) components, such as aggrecan and type II collagen, and secretion of cytokines.13, 14, 15 Importantly, deregulation of NP cell functions, including aberrant cell proliferation, apoptosis and ECM synthesis/degradation have all been implicated in the development of IDD.13, 16, 17, 18, 19, 20
Long non‐coding RNAs (lncRNAs) are a group of non‐coding RNAs which are longer than 200 nucleotides in length. Due to their long length, lncRNAs possess unique ability to adopt a variety of complex secondary and tertiary structures, allowing them to perform specific functions, such as catalysis, metabolite sensing and precise protein recognition.21, 22, 23, 24 The predicted secondary structure of Xist, which is the best‐characterised lncRNA to date, is shown in Figure 1 as an example of structural complexity of lncRNAs. LncRNAs have no or little capacity for protein coding, but could modulate gene expression at epigenetic (eg, DNA methylation, histone modification), transcriptional (eg, recruitment of transcriptional factors) and post‐transcriptional (eg, sponging of microRNAs, regulation of mRNA stability) levels.25, 26, 27, 28, 29 An increasing number of studies have demonstrated lncRNA deregulation in different kinds of morbidities, including neoplastic, inflammatory, infectious and orthopaedic diseases.30, 31, 32, 33, 34, 35 As a key control of gene expression, lncRNAs play pivotal roles in regulating cellular phenotypes, including differentiation, apoptosis, proliferation, metabolism, migration and invasion.29, 36, 37, 38, 39 Recently, some studies suggested that lncRNAs are critical players in the development of IDD. In this review article, we summarise the current knowledge regarding the deregulation of lncRNAs in IDD in relation to their effects on NP cell proliferation, apoptosis and ECM synthesis/degradation. The potential clinical utilities of lncRNAs as therapeutic targets for the management of IDD are also discussed.
Figure 1.
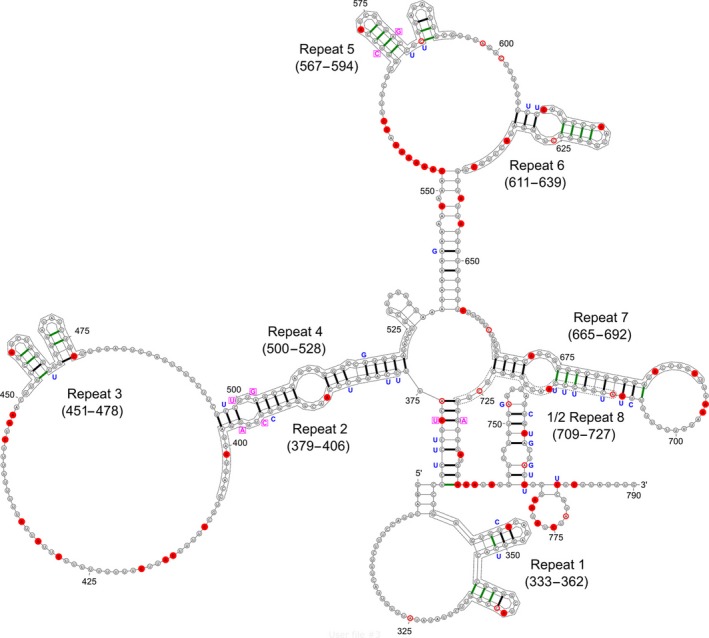
Predicted secondary structure of Xist, the best‐characterised lncRNA to date (adapted from Wikimedia Commons)
2. LncRNA EXPRESSION PROFILING IN INTERVERTEBRAL DISC DEGENERATION
Genome‐wide lncRNA profiling by microarray or RNA sequencing followed by validation of candidate lncRNAs with reverse transcription‐quantitative PCR (RT‐qPCR) is a common approach to identify and confirm differential lncRNA expression in a specific disease state.40, 41, 42 The samples analysed often include clinical specimens and cultured cells.43, 44
Wan and colleagues45 used the lncRNA‐mRNA microarray to profile differentially expressed lncRNAs and mRNAs in NP cells isolated from degenerative and non‐degenerative IVD samples. A total of 116 lncRNAs (67 upregulated and 49 downregulated) and 260 mRNAs were found to be significantly differentially expressed with fold‐change larger than ten. KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway and lncRNA‐mRNA co‐expression network analyses showed that the 10 most significantly deregulated lncRNAs were involved in several known degenerative alterations, such as chondrocyte differentiation, collagen fibril organisation and proteoglycan metabolism. In particular, by RT‐qPCR validation, the authors found that Fas‐associated protein factor‐1, which potentiates the Fas‐induced apoptosis, and its nearby enhancer‐like lncRNA RP11‐296A18.3 were both upregulated in the degenerative IVDs, implicating the involvement of RP11‐296A18.3 in aberrant NP cell apoptosis. The same team46 subsequently conducted an integrative analysis on datasets from mRNA, miRNA, circular RNA and lncRNA microarrays and further depicted the comprehensive landscape of RNAs in human IDD by illustrating the interactions among members from different RNA classes during the disease development. Chen and colleagues47 also re‐analysed the datasets from these two studies. A total of 135 upregulated and 170 downregulated lncRNAs together with 2133 upregulated and 1098 downregulated mRNAs were identified, among which seven lncRNAs (LINC00917, CTC‐523E23.5, CTD‐2246P4.1, RP11‐363G2.4, RP4‐639J15.1, RP11‐38F22.1 and AC005082.12) were predicted to play key functional roles by lncRNA‐mRNA co‐expression network analysis, in particular regulation of cell migration by interactions of CTD‐2246P4.1 and LINC00917 with SPHK1.
Unbiased genome‐wide transcriptome analysis by high‐throughput sequencing has depicted the RNA landscapes of different human tissues at unprecedented resolutions. Zhao and colleagues48 performed transcriptome sequencing on an Illumina platform to identify deregulated lncRNAs in central NP tissues isolated from IDD patients as compared with those from control patients. A total of 1854 lncRNAs were found to be differentially expressed with fold‐change more than two, in which 1530 lncRNAs were predicted to target 6386 genes through cis‐regulatory effects. KEGG pathway analysis for these target genes revealed that lncRNAs were involved in regulation of focal adhesion, lysosome function and mitogen‐activated protein kinase (MAPK) signalling. Bioinformatic analysis of interactions among lncRNAs, microRNAs, and mRNAs suggested that the upregulated lncRNA PART1 might mediate the pathogenic effect via sponging miR‐34a and miR‐148a to derepress their corresponding target genes E2F3, VEGFA and ACVR1 in IDD.
The abovementioned studies have unequivocally demonstrated that lncRNA expression is significantly deregulated in degenerative IVD tissues and NP cells and strongly suggested that lncRNAs might be functionally involved in IDD pathogenesis (Table 1). Nevertheless, it is noteworthy that different studies have given rise to different results. For instance, the top 10 upregulated and downregulated lncRNAs identified in the RNA sequencing study by Zhao and colleagues48 showed no overlap with the top deregulated lncRNAs (P < .05; fold‐change > 10) identified in the microarray study by Wan and colleagues.45 Whether the discrepancy arose as a result of the use of different clinical tissues or profiling platforms, however, is unclear.
Table 1.
Long non‐coding RNA expression profiles in intervertebral disc degeneration
| No. | Method | Sample | Filtering criteria | Upregulated | Downregulated | Reference |
|---|---|---|---|---|---|---|
| 1 | Microarray (GSE56081) RT‐PCR | Degenerate disc tissues | P < .05; Fold‐change > 10 | 67 lncRNAs | 49 lncRNAs | 45 |
| 2 | Integrative analysis of mRNA, miRNA, circular RNA and lncRNA (GSE56081) microarrays | Degenerate disc tissues | P < .05; Fold‐change > 2 | 2234 lncRNAs | 847 lncRNAs | 46 |
| 3 | Bioinformatics re‐analysis of dataset GSE56081 | Degenerate disc tissues | P < .05; Fold‐change > 1.5 | 135 lncRNAs | 170 lncRNAs | 47 |
| 4 | RNA sequencing | Degenerate disc tissues | Fold‐change > 2 | 916 lncRNAs | 938 lncRNAs | 48 |
3. FUNCTIONAL ROLES OF LNCRNAS IN INTERVERTEBRAL DISC DEGENERATION
3.1. RP11‐296A18.3
Aberrant proliferation of NP cells is implicated in IDD pathogenesis.19 Wang and colleagues49 determined the functional role of RP11‐296A18.3, an aberrantly upregulated lncRNA, in the development of IDD. The authors demonstrated that knockdown of RP11‐296A18.3 suppressed the human NP cell proliferation and reduced the expression of collagen I and matrix metalloproteinase (MMP)‐13. Target prediction, RT‐qPCR and luciferase assays further revealed that RP11‐296A18.3 acted as a competing endogenous RNA for miR‐138 and thereby derepressing its target HIF1A. In addition, the interrelationship among RP11‐296A18.3, miR‐138 and HIF1A was confirmed in human IDD tissues by immunochemistry and RT‐qPCR. These data indicated that RP11‐296A18.3 promoted human NP cell proliferation and modulated ECM deposition via regulating the miR‐138‐HIF1A axis. These results also suggested that this lncRNA might be a therapeutic target in IDD.
3.2. TUG1
Over‐activation of Wnt/β‐catenin signalling is casually linked to IDD development through multiple mechanisms, including induction of cellular senescence and abnormal ECM deposition/degradation.50 The taurine upregulated gene 1 (TUG1) is a newly identified lncRNA frequently upregulated in human malignancies30 and mechanistically linked to Wnt/β‐catenin activation.51 Chen and colleagues52 investigated the expression of lncRNA TUG1 in degenerative NP tissues and its functional role of in IDD in relation to Wnt/β‐catenin signalling. The authors first collected 30 NP samples from patients with lumbar disc herniation and 18 NP tissues from control subjects with lumbar spine trauma. TUG1 expression was found to be significantly upregulated in lumbar disc herniation NP samples and positively associated with Wnt1 and β‐catenin expression. In NP cells, enforced expression of TUG1 increased the expression of Wnt1 and β‐catenin and enhanced tumour necrosis factor (TNF)‐α‐induced cell senescence and apoptosis. Concordantly, TUG1 increased the expression of pro‐apoptotic Bax and caspase‐3 but reduced the expression of anti‐apoptotic Bcl‐2. In addition, overexpression of TUG1 upregulated ECM‐degrading genes (ie, MMP3 and ADAMTS5) but downregulated ECM‐coding genes (ie, aggrecan and COL2A1). The pro‐senescence, pro‐apoptotic and ECM‐modulating effects of TUG1 overexpression were blocked by the Wnt/β‐catenin inhibitor XAV‐939. Knockdown of TUG1 produced the opposite effects. These data suggested that aberrant upregulation of TUG1 in NP cells resulted in senescence, apoptosis and suppression of ECM synthesis through the Wnt/β‐catenin pathway. Targeting TUG1 might thus be a potential therapeutic strategy for the treatment of IDD.
3.3. HCG18
Xi and colleagues53 demonstrated that the expression of the lncRNA HCG18 (HLA complex group 18) transcribed from chromosome 6 was significantly upregulated in NP tissues isolated from patients with herniated or bulging discs and was positively associated with the grade of disc degeneration. Mechanistically, HCG18 was found to function as an endogenous sponge for miR‐146a‐5p to inhibit cell proliferation, promote cell apoptosis, and enhance release of chemoattractants for macrophages in NP cells. Conversely, knockout of miR‐146a‐5p expression produced the opposite effects. Mechanistically, TRAF6, an upstream positive regulator of nuclear factor (NF)‐κB signalling and a direct target gene of miR‐146a‐5p, and phosphorylation of NF‐κB p65 at Serine 536 were found to be upregulated upon HCG18 overexpression. The positive correlation between HCG18 and TRAF6 expression was also confirmed in clinical NP tissues. To this end, knockdown of TRAF6 by small hairpin RNA reversed HCG18‐induced NP proliferative arrest and apoptosis. These results suggested that aberrant overexpression of HCG18 might promote IDD development through inducing human NP cell death via derepressing the TRAF6‐NFκB pathway that is normally inhibited by miR‐146a‐5p.
3.4. MALAT1
MALAT1 (metastasis associated lung adenocarcinoma transcript 1) also known as NEAT2 is a lncRNA with physiological functions in different cellular processes, including alternative splicing, nuclear organisation and epigenetic modulation of gene expression.54 Zhang and colleagues55 first demonstrated that the expression level of MALAT1 was significantly reduced in NP cells isolated from IDD patients as compared with control. Functionally, restored expression of MALAT1 promoted caspase 3 activity (an apoptotic marker) and reduced the secretion of interleukins (IL)‐1 and ‐6. These findings suggested that reduced MALAT1 expression might participate in IDD development via inducing NP cell apoptosis and secretion of pro‐inflammatory cytokines.
3.5. SNHG1
Small nucleolar RNA host gene 1 (SNHG1) is a host to eight small nucleolar RNAs. The overexpression of SNHG1 has been demonstrated in hepatocellular carcinoma, lung cancer, oesophageal cancer and colorectal cancer and was associated with poorer prognosis or more aggressive phenotypes. The mechanisms involved in the oncogenic action of SHNG1 include sponging of microRNAs (eg, miR‐101‐3p, and miR‐195, miR‐338) and activation of Wnt/β‐catenin signalling.56, 57 Recently, Tan and colleagues58 investigated the role of SNHG1 in the progression of IDD. They first demonstrated that SNHG1 expression was significantly upregulated in IDD tissues as compared with control samples. Higher expression of SNHG1 was also positively correlated with the grade of disc degeneration. Functionally, enforced expression of SNHG1 induced the NP cell proliferation and promoted the cyclin D1 and PCNA expression. Mechanistically, SNHG1 repressed miR‐326 to promote NP cell proliferation. The negative correlation between SNHG1 and miR‐326 was also demonstrated in clinical IDD samples. These results suggested that the lncRNA SNHG1 played a crucal role in IDD development through inducing aberrant NP cell proliferation.
3.6. H19
H19 is an imprinted oncofoetal lncRNAs with high expression levels during embryogenesis but is nearly undetectable in most adult tissues.59 Wang and colleagues60 demonstrated that the expression of H19 was upregulated in clinical IDD specimens and in cultured NP cells undergoing oxidative stress‐induced cellular senescence. Functionally, enforced expression of H19 increased oxidative stress‐induced degeneration through inducing NP cell senescence, enhancing MMP and ADAMTS‐5 protein expression as well as inhibiting the NP cell proliferation via activating Wnt/β‐catenin signalling. To this end, H19 directly sponged miR‐22 via interacting with the 3′‐untranslated region to alleviate its inhibition on LEF1, which is a transcription factor required for β‐catenin‐driven transcription of Wnt target genes.
3.7. NEAT1
NEAT1 (nuclear paraspeckle assembly transcript 1) is a novel lncRNA localised specifically to nuclear paraspeckles and its overexpression has been documented in many human malignancies.61 Ruan and colleagues62 assessed the functional role and the associated mechanism of NEAT1 in IDD development. The authors first demonstrated that that the expression levels of NEAT1, p21 and p53 were all upregulated in the degenerative NP tissues as compared with the control NP samples. In this regard, enforced expression of NEAT1 upregulated the ECM‐degrading ADAMTS5 and MMP13 expression and suppressed aggrecan and collagen II expression through the MAPK/extracellular signal‐regulated kinase (ERK) signalling. These findings suggested that the lncRNA NEAT1 might contribute to IDD development by tipping the balance between ECM deposition and degradation by NP cells in an MAPK/ERK‐dependent manner.
3.8. linc‐ADAMTS5
Inflammation has been linked to increased aggrecan degradation in IDD.63 Wang and colleagues64 investigated the functional role of linc‐ADAMTS5, which is a lncRNA transcribed in opposite direction to the ADAMTS5 (a gene encoding an aggrecan‐degrading enzyme), in IDD. The authors found that the expression of linc‐ADAMTS5 was progressively downregulated with advancing grades of degeneration in human IDD. Bioinformatic analysis, RNA immunoprecipitation, in vitro binding assay and functional studies showed that linc‐ADAMTS5 physically interacted with the splicing factor proline/glutamine‐rich (SFPQ) to facilitate the recruitment of RREB1 (a transcription factor) to the binding site within ADAMTS5 promoter to promote chromatin remodelling and thus epigenetic silencing. Concordantly, linc‐ADAMTS5 and RREB1 expression were negatively correlated with ADAMTS5 expression in clinical NP samples. These data suggested that progressive downregulation of linc‐ADAMTS5 could promote aggrecan degradation in IDD development via derepressing the expression of ADAMTS5 (Table 2).
Table 2.
Functional characterisation of the lncRNAs in IDD
| LncRNAs | Expression | Functional role | Related genes | Reference |
|---|---|---|---|---|
| RP11‐296A18.3 | Up | Proliferation matrix metalloproteinase | miR‐138 HIF1A | 19 |
| TUG1 | Up | Senescence apoptosis ECM‐degrading | Wnt β‐catenin | 52 |
| HCG18 | Up | Proliferation apoptosis | miR‐146a‐5p TRAF6 | 53 |
| MALAT1 | Down | Apoptosis inflammatory cytokines | 55 | |
| SNHG1 | Up | Proliferation | miR‐326 | 58 |
| H19 | Up | Senescence ECM‐degrading | miR‐22 LEF1 | 60 |
| NEAT1 | Up | ECM‐degrading | ERK/MAPK | 62 |
| linc‐ADAMTS5 | Down | Aggrecan degradation | ADAMTS5 | 64 |
4. CONCLUSION
Intervertebral disc degeneration is a common pathogenic process underlying low back pain. However, its molecular and cellular pathogenesis remains largely unknown. Emerging evidence supported that lncRNAs play critical roles in orthopaedic diseases. In particular, an increasing number of studies have hinted at the functional roles of lncRNAs in IDD and have shed new light on their clinical use as potential therapeutic targets. Through lncRNA profiling and RT‐PCR, several key deregulated lncRNAs in both NP cells and tissues have been identified. However, functional studies of these lncRNAs still remain limited. LncRNAs might be involved in IDD initiation and progression through modulating NP cell proliferation, apoptosis and ECM deposition (Figure 2). Deregulated lncRNAs might also alter cytokines release (eg, IL‐1, IL‐6) or modulate response to cytokines (eg, TNF‐α‐induced cell senescence and apoptosis) in NP cells and thereby contributing to inflammation. Treatment for IDD might thus be achieved through targeting deregulated lncRNAs, for example, using lncRNA‐specific small interfering RNA delivered by nanoparticles or lipid‐encapsulation as well as small‐molecule inhibitors that perturb that interaction of particular lncRNA with its RNA or protein partners. Apart from treatment, early detection and prognostication of IDD remain clinically challenging. It is noteworthy that certain circulating or tissue lncRNAs have been proposed as novel diagnostic and prognostic markers in human cancers. Nevertheless, systematic identification and validation of lncRNAs as biomarkers in IDD are still lacking. More translational works are thus needed to maximise the clinical potentials of lncRNAs in IDD.
Figure 2.
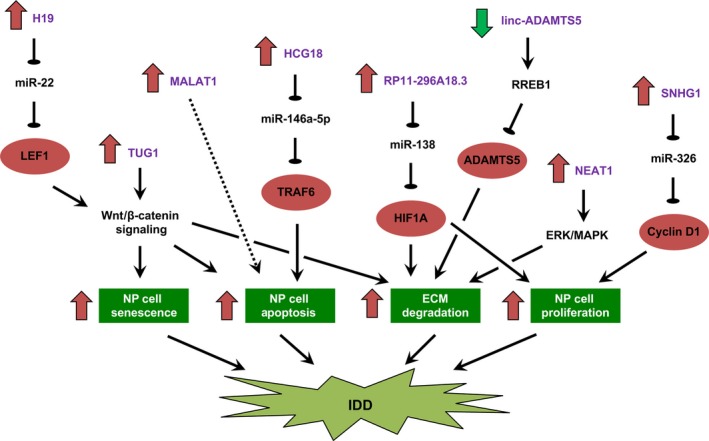
Deregulation of intracellular signalling and functions of nucleus pulposus cells by lncRNAs in intervertebral disc degeneration
Over the recent years, a rapid expansion of technologies has greatly accelerated the discovery and functional characterisation of disease‐associated lncRNAs. These technological advancements with respect to lncRNA profiling interactome analysis (comprehensively reviewed by Jathar et al65) have promoted the identification and mechanistic study of lncRNAs in other diseases. It is anticipated these methods could be applied to the study of IDD‐associated lncRNAs and shed new light on the molecular pathogenesis of IDD.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (Grant Numbers: 81330044) and The Capital Health Research and Development of Special (Grant Numbers: 2016‐1‐4096).
Li Z, Li X, Chen C, et al. Long non‐coding RNAs in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2018;51:e12483 10.1111/cpr.12483
Zheng Li, Xingye Li and Chong Chen are the co‐first authors.
Contributor Information
Shugang Li, Email: lishugangpumc@163.com.
Jianxiong Shen, Email: sjxpumch@163.com.
REFERENCES
- 1. Becker A, Held H, Redaelli M, et al. Implementation of a guideline for low back pain management in primary care ‐ a cost‐effectiveness analysis. Spine. 2012;37:701‐710. [DOI] [PubMed] [Google Scholar]
- 2. Samartzis D, Karppinen J, Mok F, Fong DY, Luk KD, Cheung KM. A population‐based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 2011;93:662‐670. [DOI] [PubMed] [Google Scholar]
- 3. Tajerian M, Alvarado S, Millecamps M, et al. DNA methylation of SPARC and chronic low back pain. Mol Pain. 2011;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Speed C. Low back pain. BMJ. 2004;328:1119‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arana E, Kovacs FM, Royuela A, et al. Modic changes and associated features in Southern European chronic low back pain patients. Spine J. 2011;11:402‐411. [DOI] [PubMed] [Google Scholar]
- 6. Tegeder I, Lotsch J. Current evidence for a modulation of low back pain by human genetic variants. J Cell Mol Med. 2009;13(8B):1605‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Omair A, Holden M, Lie BA, Reikeras O, Brox JI. Treatment outcome of chronic low back pain and radiographic lumbar disc degeneration are associated with inflammatory and matrix degrading gene variants: a prospective genetic association study. BMC Musculoskelet Disord. 2013;14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loreto C, Musumeci G, Castorina A, Martinez G. Degenerative disc disease of herniated intervertebral discs is associated with extracellular matrix remodeling, vimentin‐positive cells and cell death. Ann Anat. 2011;193:156‐162. [DOI] [PubMed] [Google Scholar]
- 9. Phillips KL, Jordan‐Mahy N, Nicklin MJ, Le Maitre CL. Interleukin‐1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann Rheum Dis. 2013;72:1860‐1867. [DOI] [PubMed] [Google Scholar]
- 10. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10‐14. [DOI] [PubMed] [Google Scholar]
- 11. Inoue N, Espinoza Orias AA. Biomechanics of intervertebral disk degeneration. Orthop Clin North Am. 2011;42:487‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151‐2161. [DOI] [PubMed] [Google Scholar]
- 13. Li Z, Yu X, Shen J, Chan MT, Wu WK. MicroRNA in intervertebral disc degeneration. Cell Prolif. 2015;48:278‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang SZ, Rui YF, Lu J, Wang C. Cell and molecular biology of intervertebral disc degeneration: current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014;47:381‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gruber HE, Ingram JA, Leslie K, Hanley EN Jr. Cellular, but not matrix, immunolocalization of SPARC in the human intervertebral disc: decreasing localization with aging and disc degeneration. Spine. 2004;29:2223‐2228. [DOI] [PubMed] [Google Scholar]
- 16. Li HR, Cui Q, Dong ZY, Zhang JH, Li HQ, Zhao L. Downregulation of miR‐27b is involved in loss of type II collagen by directly targeting matrix metalloproteinase 13 (MMP13) in human intervertebral disc degeneration. Spine. 2016;41:E116‐E123. [DOI] [PubMed] [Google Scholar]
- 17. Li Z, Shen J, Wu WK, et al. Leptin induces cyclin D1 expression and proliferation of human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK pathways. PLoS ONE. 2012;7:e53176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Li Z, Yu X, Liang J, Wu WK, Yu J, Shen J. Leptin downregulates aggrecan through the p38‐ADAMST pathway in human nucleus pulposus cells. PLoS ONE. 2014;9:e109595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Yu X, Li Z, Shen J, et al. MicroRNA‐10b promotes nucleus pulposus cell proliferation through RhoC‐Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS ONE. 2013;8:e83080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Li Z, Shen J, Wu WK, et al. The role of leptin on the organization and expression of cytoskeleton elements in nucleus pulposus cells. J Orthop Res. 2013;31:847‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu X, Li Z. Long non‐coding RNA HOTAIR: a novel oncogene (Review). Mol Med Rep. 2015;12:5611‐5618. [DOI] [PubMed] [Google Scholar]
- 22. Yu X, Li Z. Long non‐coding RNA growth arrest‐specific transcript 5 in tumor biology. Oncol Lett. 2015;10:1953‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Z, Yu X, Shen JX. Long non‐coding RNAs: emerging players in osteosarcoma. Tumor Biol. 2016;37:2811‐2816. [DOI] [PubMed] [Google Scholar]
- 24. Huang CS, Liu SG, Wang HJ, Zhang ZC, Yang Q, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8:5025‐5034. [PMC free article] [PubMed] [Google Scholar]
- 25. Li P, Xue WJ, Feng Y, Mao QS. Long non‐coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res. 2016;8:3522‐3529. [PMC free article] [PubMed] [Google Scholar]
- 26. Liu CB, Lin JJ. Long noncoding RNA ZEB1‐AS1 acts as an oncogene in osteosarcoma by epigenetically activating ZEB1. Am J Transl Res. 2016;8:4095‐4105. [PMC free article] [PubMed] [Google Scholar]
- 27. Ma GX, Tang MQ, Wu YQ, Xu XM, Pan F, Xu RA. LncRNAs and miRNAs: potential biomarkers and therapeutic targets for prostate cancer. Am J Transl Res. 2016;8:5141‐5150. [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Li Z, Zheng W, et al. LncRNA‐ATB: an indispensable cancer‐related long noncoding RNA. Cell Prolif. 2017;50(6). 10.1111/cpr.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J, Lian Y, Yan C, et al. Long non‐coding RNA FOXP4‐AS1 is an unfavourable prognostic factor and regulates proliferation and apoptosis in colorectal cancer. Cell Prolif. 2017;50(1). 10.1111/cpr.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li Z, Shen J, Chan M, Wu W. TUG1: a pivotal oncogenic long non‐coding RNA of human cancers. Cell Prolif. 2016;49:471‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma X, Qi S, Duan Z, et al. Long non‐coding RNA LOC554202 modulates chordoma cell proliferation and invasion by recruiting EZH2 and regulating miR‐31 expression. Cell Prolif. 2017;50(6). 10.1111/cpr.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao J, Gao Z, Zhang C, Wu H, Gu R, Jiang R. Long non‐coding RNA ASBEL promotes osteosarcoma cell proliferation, migration and invasion by regulating microRNA‐21. J Cell Biochem. 2018; 10.1002/jcb.26671. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33. Zhang X, Tang X, Ma L, et al. Schisandrin B down‐regulated lncRNA BCYRN1 expression of airway smooth muscle cells by improving miR‐150 expression to inhibit the proliferation and migration of ASMC in asthmatic rats. Cell Prolif. 2017;50(6). 10.1111/cpr.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei B, Wei W, Zhao B, Guo X, Liu S. Long non‐coding RNA HOTAIR inhibits miR‐17‐5p to regulate osteogenic differentiation and proliferation in non‐traumatic osteonecrosis of femoral head. PLoS ONE. 2017;12:e0169097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Runtsch MC, O'Neill LAJ. GOTcha: lncRNA‐ACOD1 targets metabolism during viral infection. Cell Res. 2018;28:137‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang C, Wu K, Wang S, Wei G. Long non‐coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR‐195‐5p. J Cell Biochem. 2018;119(7):5646‐5656. [DOI] [PubMed] [Google Scholar]
- 37. Zhang YH, Fu J, Zhang ZJ, Ge CC, Yi Y. LncRNA‐LINC00152 down‐regulated by miR‐376c‐3p restricts viability and promotes apoptosis of colorectal cancer cells. Am J Transl Res. 2016;8:5286‐5297. [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao HX, Hou WG, Tao JG, et al. Upregulation of lncRNA HNF1A‐AS1 promotes cell proliferation and metastasis in osteosarcoma through activation of the Wnt/beta‐catenin signaling pathway. Am J Transl Res. 2016;8:3503‐3512. [PMC free article] [PubMed] [Google Scholar]
- 39. Zou ZW, Ma C, Medoro L, et al. LncRNA ANRIL is up‐regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side‐population stem‐like cancer cells. Oncotarget. 2016;7:61741‐61754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Su Y, Li L, Zhao S, Yue YN, Yang SX. The long noncoding RNA expression profiles of paroxysmal atrial fibrillation identified by microarray analysis. Gene. 2018;642:125‐134. [DOI] [PubMed] [Google Scholar]
- 41. Liu H, Dai CF, Wu QR, Liu HY, Li FT. Expression profiling of long noncoding RNA identifies lnc‐MMP3‐1 as a prognostic biomarker in external auditory canal squamous cell carcinoma. Cancer Med. 2017;6:2541‐2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang WY, Dong R, Diao S, Du J, Fan ZP, Wang F. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu CY, Liu H, Zhang FE, et al. Long noncoding RNA expression profile reveals lncRNAs signature associated with extracellular matrix degradation in kashin‐beck disease. Sci Rep. 2017;7:17553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deng BY, Cheng X, Li HM, Qin JB, Tian ML, Jin GH. Microarray expression profiling in the denervated hippocampus identifies long noncoding RNAs functionally involved in neurogenesis. BMC Mol Biol. 2017;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wan ZY, Song F, Sun Z, et al. Aberrantly expressed long noncoding RNAs in human intervertebral disc degeneration: a microarray related study. Arthritis Res Ther. 2014;16:465 10.1186/S13075-014-0465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lan PH, Liu ZH, Pei YJ, et al. Landscape of RNAs in human lumbar disc degeneration. Oncotarget. 2016;7:63166‐63176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen Y, Ni HJ, Zhao YC, et al. Potential role of lncRNAs in contributing to pathogenesis of intervertebral disc degeneration based on microarray data. Med Sci Monitor. 2015;21:3449‐3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao B, Lu M, Wang D, Li H, He X. Genome‐wide identification of long noncoding RNAs in human intervertebral disc degeneration by RNA sequencing. Biomed Res Int. 2016;2016:3684875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang XB, Lv GH, Li J, Wang B, Zhang QS, Lu C. LncRNA‐RP11‐296A18.3/miR‐138/HIF1A pathway regulates the proliferation ecm synthesis of human nucleus pulposus cells (HNPCs). J Cell Biochem. 2017;118:4862‐4871. [DOI] [PubMed] [Google Scholar]
- 50. Hiyama A, Sakai D, Risbud M, et al. Enhancement of intervertebral disc cell senescence by WNT/β‐catenin signaling‐induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036‐3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liang S, Zhang S, Wang P, et al. LncRNA, TUG1 regulates the oral squamous cell carcinoma progression possibly via interacting with Wnt/β‐catenin signaling. Gene. 2017;608:49‐57. [DOI] [PubMed] [Google Scholar]
- 52. Chen J, Jia YS, Liu GZ, et al. Role of LncRNA TUG1 in intervertebral disc degeneration and nucleus pulposus cells via regulating Wnt/beta‐catenin signaling pathway. Biochem Biophys Res Comm. 2017;491:668‐674. [DOI] [PubMed] [Google Scholar]
- 53. Xi YH, Jiang TW, Wang WH, et al. Long non‐coding HCG18 promotes intervertebral disc degeneration by sponging miR‐146a‐5p and regulating TRAF6 expression. Sci Rep. 2017;7(1):13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang X, Hamblin M, Yin K. The long noncoding RNA Malat 1: its physiological and pathophysiological functions. RNA Biol. 2017;14:1705‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang H, Li JD, Duan DP, She W, Wang LG, Zhang FQ. The role of lncRNA MALAT1 in intervertebral degenerative disc disease. Int J Clin Exp Pathol. 2017;10:10611. [PMC free article] [PubMed] [Google Scholar]
- 56. Cui Y, Zhang F, Zhu C, Geng L, Tian T, Liu H. Upregulated lncRNA SNHG1 contributes to progression of non‐small cell lung cancer through inhibition of miR‐101‐3p and activation of Wnt/β‐catenin signaling pathway. Oncotarget. 2017;8:17785‐17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tian T, Qiu R, Qiu X. SNHG1 promotes cell proliferation by acting as a sponge of miR‐145 in colorectal cancer. Oncotarget. 2018;9:2128‐2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tan H, Zhao L, Song R, Liu Y, Wang L. The long noncoding RNA SNHG1 promotes nucleus pulposus cell proliferation through regulating miR‐326/CCND1. Am J Physiol Cell Physiol. 2018; 10.1152/ajpcell.00220.2017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 59. Raveh E, Matouk I, Gilon M, Hochberg A. The H19 Long non‐coding RNA in cancer initiation, progression and metastasis ‐ a proposed unifying theory. Mol Cancer. 2015;14:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang X, Zou M, Li J, et al. LncRNA H19 targets miR‐22 to modulate HO‐induced deregulation in nucleus pulposus cell senescence, proliferation, and ECM synthesis through Wnt signaling. J Cell Biochem. 2018;119(6):4990‐5002. [DOI] [PubMed] [Google Scholar]
- 61. Yu X, Li Z, Zheng H, Chan M, Wu W. NEAT1: a novel cancer‐related long non‐coding RNA. Cell Prolif. 2017;50(2). 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ruan Z, Ma H, Li J, Liu H, Jia H, Li F. The long non‐coding RNA NEAT1 contributes to extracellular matrix degradation in degenerative human nucleus pulposus cells. Exp Biol Med (Maywood). 2018;243:595‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun Z, Yin Z, Liu C, Liang H, Jiang M, Tian J. IL‐1β promotes ADAMTS enzyme‐mediated aggrecan degradation through NF‐κB in human intervertebral disc. J Orthop Surg Res. 2015;10:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang K, Song Y, Liu W, et al. The noncoding RNA linc‐ADAMTS5 cooperates with RREB1 to protect from intervertebral disc degeneration through inhibiting ADAMTS5 expression. Clin Sci. 2017;131:965‐979. [DOI] [PubMed] [Google Scholar]
- 65. Jathar S, Kumar V, Srivastava J, Tripathi V. Technological Developments in lncRNA Biology In: Rao M, ed. Long Non Coding RNA Biology. Advances in Experimental Medicine and Biology. Vol 1008. Singapore: Springer; 2017;1008:283‐323. [DOI] [PubMed] [Google Scholar]


