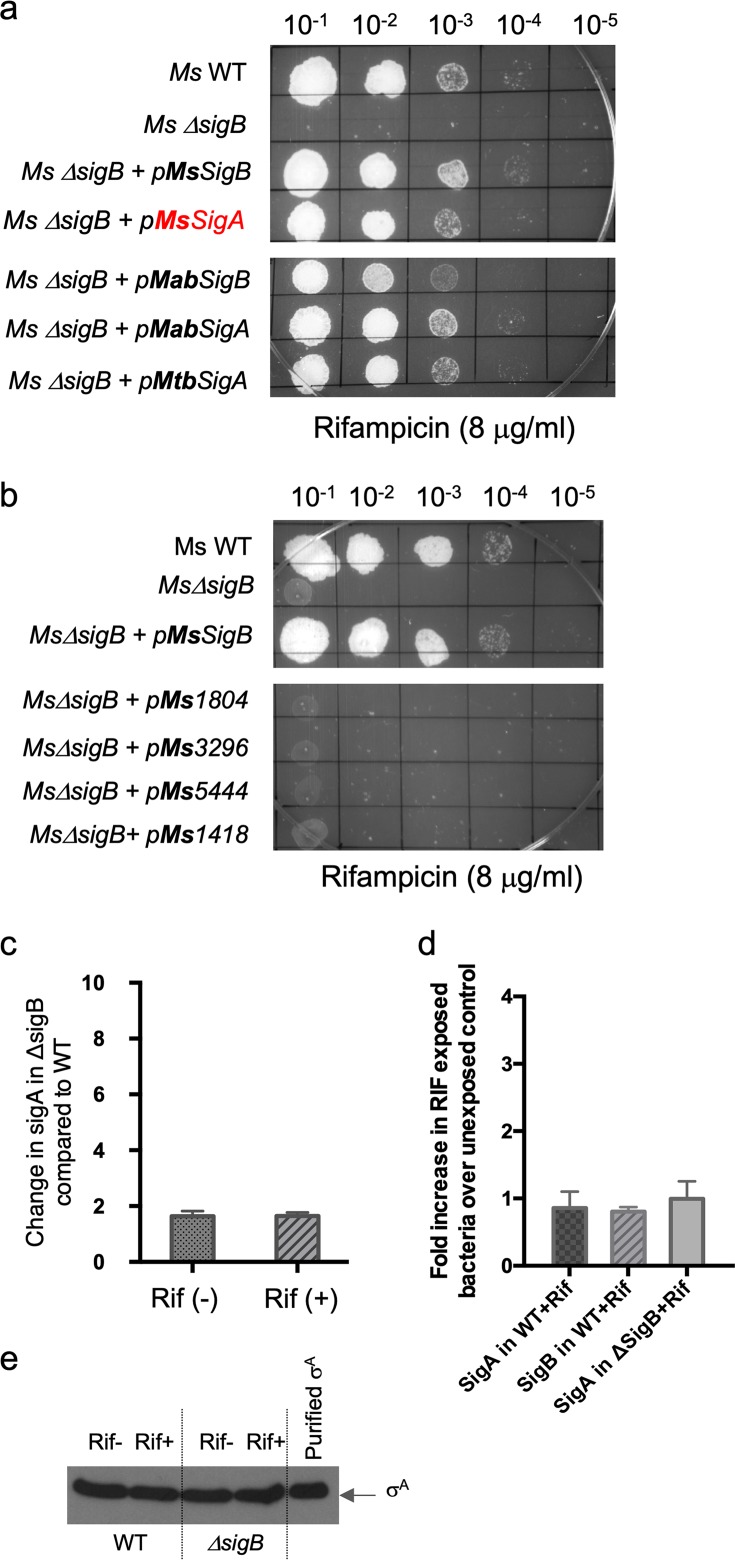
FIG 6.
Overexpression of σA restores the RIF sensitivity of the MsΔsigB mutant. (a to c) Complemented strains were created by integrating Ms_SigA, Mtb_SigA, Mab_SigA, Ms_SigB, Mtb_SigB, Mab_SigB, Ms_Ms1804, Ms_Ms3296, Ms_Ms5444, and MSMEG_1418 at the Bxb1 attB site of mc2155 ΔsigB. Tenfold serial dilutions of M. smegmatis mc2155, the mc2155 ΔsigB mutant, and the complemented strains were grown to an A600 of 0.7 and spotted on Middlebrook 7H10 ADC plates containing the indicated concentrations of RIF. The RIF sensitivity of mc2155 ΔsigB could be complemented by the constitutive expression of sigA and sigB from all mycobacterial strains but not by ECF sigma factors. (b) Wild-type M. smegmatis and the MsΔsigB strain were grown to an A600 of 0.7 and exposed to 4 μg/ml RIF for 30 min, and the amount of the M. smegmatis sigA transcript was determined by qPCR and plotted as the fold induction of sigA levels in the MsΔsigB strain over the level of expression in the wild-type strain. The data represent the mean ± SD (n = 3). MSMEG_4936 was used as an endogenous control, as its levels were unchanged under various conditions in RNA-seq experiments. (d) Wild-type M. smegmatis and the MsΔsigB strain were grown to an A600 of 0.7 and exposed to 4 μg/ml RIF for 30 min, and the amounts of the M. smegmatis sigA and sigB transcripts were determined by qPCR and plotted as the fold induction upon RIF exposure over the level of expression for an unexposed control. The data represent the mean ± SD (n = 3). MSMEG_4936 was used as an endogenous control. (e) Wild-type M. smegmatis and the MsΔsigB strain were grown to an A600 of 0.7 and exposed to 4 μg/ml RIF for 30 min. The levels of σA protein were determined by Western blotting using an anti-σ70 monoclonal antibody. Samples were normalized by wet weight and protein concentration to ensure equivalent loading of each sample. Purified σA was used as a control.