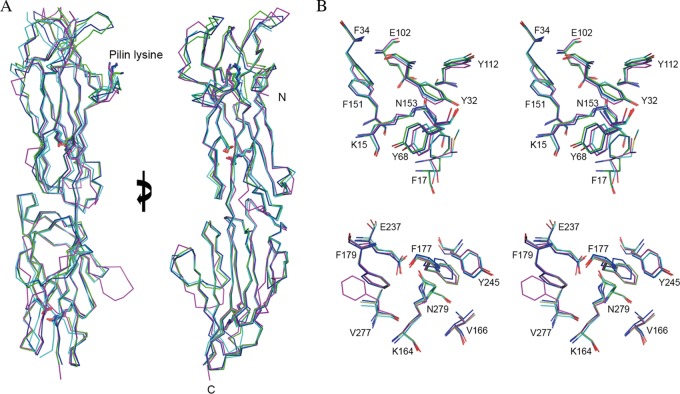
FIG 3.
Structural overlay of T antigens. (A) Ribbon diagram showing the almost identical Cα trace, with an overall RMSD of 1.34 Å over 261 aligned residues, among the four structures of T18.1 (green), T13 (cyan), T3.2 (blue), and T1 (magenta). The key residues involved in intramolecular isopeptide bond formation and the pilin lysine (K) are shown in stick mode. Views are at 0° and 90° rotations around the y axis. (B) Stereodiagram of the conserved intramolecular isopeptide bonds. The isopeptide bonds of both the N-terminal domain (top) and the C-terminal domain (bottom) are buried in the hydrophobic core of the β-clasp. For clarity, only T18.1 residues are numbered. The isopeptide bond forms between a buried lysine (K15) and asparagine (N153) in the N-terminal domain and between lysine (K164) and asparagine (N279) in the C-terminal domain. The conserved hydrophobic core surrounding the isopeptide bonds is largely composed of bulky aromatics. A strictly conserved buried glutamate (E102 and E237) is essential for isopeptide bond formation.