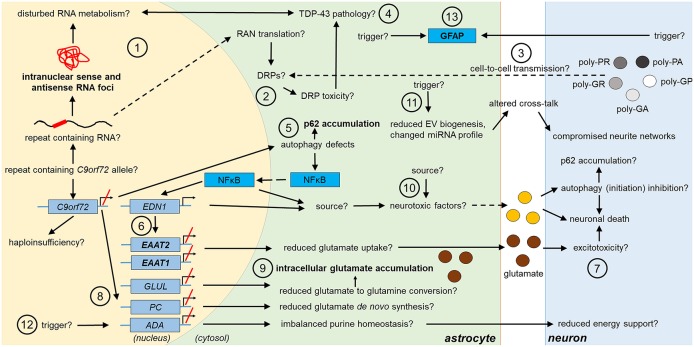
Figure 1.
Potential and confirmed phenotypic features of C9orf72 HRE-associated astrocytes in FTLD/ALS. Features detected in the astrocytes of FTLD or ALS patient post-mortem brain are indicated in bold text. The presence of the typical C9-HRE-associated pathological hallmarks, which have previously been observed mainly in neurons, as well as other potential mechanisms, which still need to be confirmed in human patient astrocytes, are indicated with a question mark. Directions of sequential events are visualized with arrows. Steps requiring intracellular or intercellular translocation of molecules are indicated by dashed arrows. The different events are indicated by numbers as follows: (1) C9-HRE-containing RNA might be transcribed in astrocytes, forming intranuclear sense and antisense RNA foci, which may disturb RNA metabolism. (2) C9-HRE-containing RNA might be translocated into the cytosol of astrocytes, where it could undergo RAN translation creating potentially toxic DRPs. (3) DRPs might also be transmitted from other cell types, such as neurons, to astrocytes. (4) Potential disturbances in RNA metabolism as well as DRP toxicity might lead to TDP-43 pathology, which in turn could lead to defects in RNA processing in astrocytes. (5) Reduced C9orf72 levels in astrocytes might cause defects in autophagy, resulting in p62 accumulation and increase of NFκB levels in the cytosol and nucleus. (6) Enhanced NFκB levels with simultaneously decreased C9orf72 levels might enhance EDN1 expression, which suppresses EAAT1 and 2 expression in astrocytes. (7) As a result, astrocytic uptake of extracellular glutamate might be diminished, which might lead to excitotoxicity. (8) Reduced C9orf72 levels might lead to decreased expression of genes involved in glutamate de novo synthesis (e.g., PC) and glutamate to glutamine conversion (e.g., GLUL). (9) Reduced conversion of glutamate to glutamine might underlie intracellular glutamate accumulation. (10) Neurotoxic factors, which partly might be created through the altered NFκB signaling, cause neuronal death. Neurotoxic factors might cause autophagy inhibition in neurons, which might lead to p62 accumulation. (11) Reduced EV synthesis, which might lead to decreased EV secretion, and an altered miRNA expression profile, might influence the crosstalk between astrocytes and neurons, which could lead to disturbed neurite growth and networks. (12) Decreased expression of ADA might disrupt purine metabolism and lead to decreased energy support of neurons by astrocytes. (13) Enhanced GFAP expression might derive from endogenous or exogenous triggers. ADA, adenosine deaminase; DRP, dipeptide repeat protein; EAAT1/2, excitatory amino acid transporter1/2; EDN1, endothelin 1; EV, extracellular vesicle; GFAP, glial fibrillary acidic protein; GLUL, glutamate-ammonia ligase; HRE, hexanucleotide repeat expansion; miRNA, micro ribonucleic acid; NFκB, nuclear factor “kappa-light-chain-enhancer” of activated B-cells; PC, pyruvate carboxylase; RAN, repeat-associated non-AUG; TDP-43, Transactive response DNA-binding protein 43.