Fig. 1.
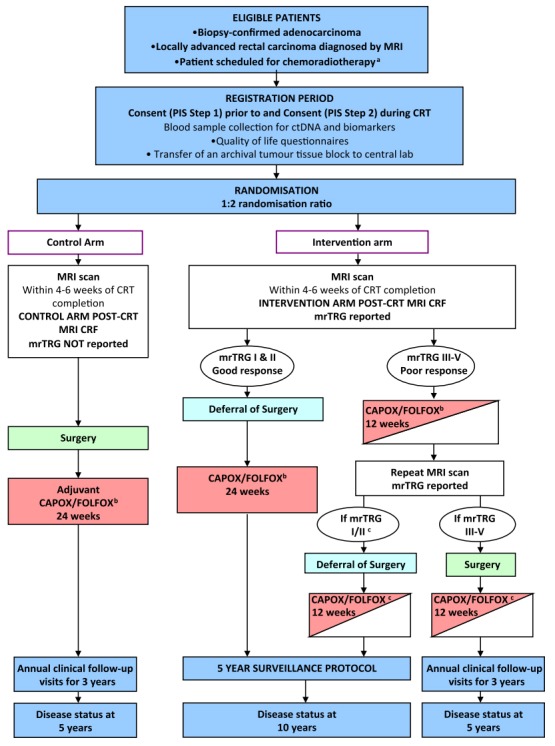
The Magnetic resonance tumour regression grade as a novel biomarker to stratify management of good and poor responders to chemoradiotherapy: a rectal cancer multicentre randomised control trial (TRIGGER trial) is a multicenter, open, interventional, randomized control feasibility study to validate assessment of tumor response based on magnetic resonance imaging (MRI)-derived tumor regression grading system, named mrTRG [65]. CRT, chemoradiotherapy; mrTRG, magnetic resonance tumor regression grade; CRF, case report form; PIS, patient information sheet; CAPOX, capecitabine with oxaliplatin; FOLFOX, 5-fluorouracil (5-FU), leucovorin, and oxaliplatin. aScheduled to receive 45 Gy–55 Gy long-course radiotherapy. bTreatment decision should be made prior to registration (planned choice is a randomisation stratification variable). Medical oncologist may choose to use CAPOX or FOLFOX, or signle-agent capecitabine or 5-FU if concomitant use of oxaliplatin is contraindicated. cPatient defers surgery then the remaining 12 weeks of chemotherapy should be given as soon as possible following the repeat MRI scan and multidisciplinary team meeting.
