Figure 1.
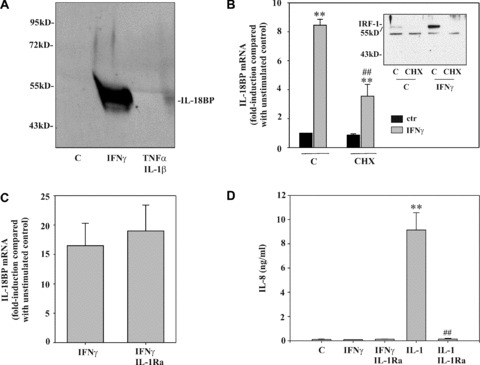
Optimal induction of IL‐18BP in DLD‐1 cells requires protein translation. (A) Cells were kept as unstimulated control, stimulated with IFN‐γ (10 ng/ml) or with IL‐1β/TNF‐α (both 20 ng/ml) for 24 hrs. TCA‐precipitated supernatants were analysed by immunoblot analysis. One representative of three independently performed experiments is shown. (B) Cells were kept as unstimulated control or stimulated with CHX (10 μg/ml), IFN‐γ (10 ng/ml), or CHX (10 μg/ml)/IFN‐γ (10 ng/ml). CHX was always added 2 hrs before IFN‐γ. After 8 hrs, IL‐18BP mRNA was evaluated by realtime PCR. IL‐18BP mRNA was normalized to that of GAPDH. Data are expressed as fold induction compared with unstimulated control ± S.D. (n= 3). **, P < 0.01 compared with unstimulated control or with cycloheximide alone; ##, P < 0.01 compared with IFN‐γ alone. Inset: DLD‐1 cells were kept as unstimulated control or stimulated as described above. After 1 hr, nuclear IRF1 was evaluated by immunoblot analysis. (C) DLD‐1 cells were kept as unstimulated control or stimulated for 12 hrs with IFN‐γ (10 ng/ml) or with IFN‐γ (10 ng/ml)/IL‐1Ra (5 μg/ml). IL‐1Ra was always added 5 hrs before IFN‐γ. IL‐18BP mRNA was evaluated by realtime PCR. IL‐18BP mRNA was normalized to that of GAPDH. Data are expressed as fold induction compared with unstimulated control ± S.D. (n= 3). (D) Supernatants generated by the experiments shown in (C) were evaluated for IL‐8 levels by ELISA. Additional conditions: IL‐1β (2 ng/ml) alone and IL‐1β (2 ng/ml)/IL‐1Ra (5 μg/ml). Data are shown as mean ± S.D. (n= 3). **, P < 0.01 compared with unstimulated control; ##, P < 0.01 compared with IL‐1β.
