Abstract
Background
Excessive activation of NLRP3 inflammasome and down-regulation of Sirt1/Smad3 deacetylation pathway play a significant role in the evolution of renal fibrosis. In China, it has been well known that Chinese herbal medicine is markedly effective in treating chronic kidney disease (CKD). Shen Shuai IIRecipe (SSR) has been used clinically for more than 20 years and has been confirmed to be effective in improvements of renal function and fibrosis. However, the specific mechanisms under the efficacy require further research. The purpose of this study was to evaluate whether SSR could alleviate renal injury and fibrosis by regulating NLRP3 inflammasome and Sirt1/Smad3 deacetylation pathway.
Methods
Four weeks after 5/6 ablation/infarction (A/I) surgery, Sprague-Dawley rats were randomly divided into the following groups: sham operation group, 5/6 (A/I) group, 5/6 (A/I) + SSR group, and 5/6 (A/I) + Losartan group (5/6 (A/I) + Los). After 8 weeks intervention,we mainly assessed the severity of renal injury and fibrosis along with the activation of NLRP3 inflammasome and Sirt1/Smad3 deacetylation pathway.
Results
SSR significantly attenuated renal injury and fibrosis in the remnant kidneys. In addition, we found that SSR effectively inhibited activation of NLRP3/ASC/Caspase-1/IL-1βcascade, decreased inflammatory infiltration and up-regulated Sirt1/Smad3 deacetylation pathway.
Conclusions
SSR could contribute to renal protection by inhibiting the activation of NLRP3 inflammasome and, furthermore, strengthen the antifibrotic effects by up-regulating Sirt1/Smad3 deacetylation pathway in 5/6 renal (A/I) model.
Keywords: Shen Shuai IIRecipe,Chronic kidney disease; NLRP3 inflammasome; Renal fibrosis; Deacetylation
Background
Chronic kidney disease (CKD) affects over 10% of the human population worldwide with high mortality, in part due to limited available or affordable treatment [1]. The prevalence of CKD is increasing worldwide and renal fibrosis is the common outcome of CKD, irrespective of the initial causes [2, 3]. The activation of renal fibroblasts and excessive accumulation of extracellular matrix (ECM) components are the hallmark of renal tubulointerstitial fibrosis,which is closely interconnected with tissue regeneration and inflammation [4].
Tubulointerstitial inflammation is a critical pathological feature of CKD and promotes the evolution of interstitial fibrosis [5].NLRP3,a NOD-like pattern recognition receptor,initiates innate immune response during tissue injury or pathogen infections [6]. Upon activation,the NLRP3 proteins undergo oligomerization and recruit the adaptor protein ASC and pro-caspase-1 to form inflammasome [7, 8]. The formation of the inflammasome facilitates the release of active caspase-1 and the subsequent secretion of mature pro-inflammatory cytokines interleukin (IL)-1β and IL-18.Previous reports suggested the NLRP3 complex could play a significant role in promoting inflammatory response and renal fibrosis in CKD [9]. Zhuang et al. [10] demonstrated activation of NLRP3 inflammasome induced by proteinuria was involved in the progressive loss of renal function in CKD. Ludwig-Portugall et al. [11]. confirmed that CP-456,773,a specific inhibitor of the NLRP3 inflammasome, attenuated crytal-induced kidney fibrosis.
Transforming growth factorβ-1 (TGFβ-1),a profibrotic factor, has been shown to implicate in activation of renal fibroblasts and accumulation of ECM proteins [12].TGF-β1 signal activates Smad2 and Smad3 phosphorylation and subsequently forms a complex with Smad4 to modulate the transcription of target genes [13, 14].However,previous studies reported acetylation of Smad3 was involved in TGFβ1-dependent renal fibrosis [15]. Sirtuin 1(Sirt1),a conserved NAD+-dependent protein deacetylase, is a survival factor that is involved in lifespan extension and has been demonstrated to deacetylate the lysine residues [16]. He et al. [17].confirmed that activation of Sirt1 attenuated kidney fibrosis in UUO model. Therefore, the inhibition of NLRP3 inflammasome and activation of Sirt1 could be attractive targets to ameliorate renal fibrosis.
Traditional Chinese medicine (TCM) has been widely used for disease prevention and treatment over many years of clinical validation and become popular for promoting healthcare [18]. In China, it has been well known that Chinese herbal medicine is markedly effective in treating CKD. Shen Shuai IIRecipe (SSR),an effective prescription for the treatment of CKD in the clinic, is composed of Epimedium,Codonopsis pilosula, Salvia miltiorrhiza bge, Rheum palmatum, Angelica sinensis, Folium perillae, Coptis chinensis, Peach kernel,and Ligusticum chuanxiong, aiming to‘fortify the spleen and tonify the kidney’and‘activate blood and resolve stasis’.Clinically, SSR has been used for more than 20 years and become the basic prescription for the treatment of patients with CKD. Previous clinical studies suggested SSR significantly improved renal function of patients with CKD, as evidenced by decreased levels of serum creatinine (Scr),24-h urinary protein quantity, blood urea nitrogen (BUN) and increased evaluated glomerular filtration rates (eGFRs) [19]. Renal ultrasound detection revealed that SSR markedly increased renal blood flow in primary CKD 3 or 4 stage patients [19]. Previous experimental studies showed that SSR significantly down-regulated the expression of NF-κB/TNF-α signaling pathway and attenuated renal fibrosis by improving oxygen consumption in 5/6 renal ablation/infarction (A/I) model [20, 21]. Importantly, We didn’t observe any side effects or changes of liver function in clinical patients and experimental animals. These findings may indicate that the therapeutic effect of SSR is devoid of any toxic manifestation. In the present study, we aimed to investigate whether SSR could alleviate renal fibrosis by regulating NLRP3 inflammasome and Sirt1/Smad3 deacetylation pathway.
Methods
Animals and drugs
Eight-week-old male Sprague-Dawley (SD) rats (190 g–210 g) were obtained from SLAC Laboratory Animal Co., Ltd. (Shanghai,China) and maintained under a controlled temperature (23 ± 3 °C) and humidity (55 ± 15%) with a 12 h light/12 h dark cycle. SSR consists of Epimedium 15 g,Codonopsis pilosula 15 g,Salvia miltiorrhiza bge 15 g,Rheum palmatum 15 g,Angelica sinensis 15 g,Folium perillae 15 g,Coptis chinensis 6 g,Peach kernel 15 g,and Ligusticum chuanxiong 15 g. The raw herbs for preparation of SSR were obtained from Shanghai Kangqiao Chinese Medicine Tablet Co.,Ltd. (Shanghai,China) and identified by Dr. Guanglin Xu from Pharmacy Department, Shuguang Hospital Affiliated to Shanghai University of TCM. The above nine commonly used Chinese herbs were mixed with distilled water in proportion and heated twice at 100 °C for 1 h under continuous stirring condition. The aqueous extracts were subjected to centrifugation at 1500×g. The supernatants were merged and concentrated to prepare suspension containing 6 g/mL of the original drug by means of ethanol. The gavage dose (10 mL/kg) in the present study was determined according to the previous studies [21]. Losartan tablets (100 mg/tablet) were obtained from MSD Pharmaceutical Co., Ltd.(Hangzhou,China) and used as positive control. Losartan was dissolved and diluted by saline with a concentration of 5 mg/mL of solution.
Rat 5/6(A/I) model and animal study protocol
5/6 (A/I) surgery was performed as described previously [21, 22]. The rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.), and placed on temperature-controlled surgical table. The surgical area was disinfected with 75% alcohol and a flank incision was then performed. The left kidney was taken out from the retroperitoneum and the renal artery of left kidney was exposed after separation of perirenal fat and renal capsular. Two branches of the left renal artery were ligated with 4–0 silk sutures and the gelatin sponge was used to suppress the bleeding for a moment. The left kidney was gently retracted back into the body and the incision was sutured. After one week,a right flank incision was performed. After separating the adrenal gland, the right renal pedicle was ligated and the kidney was removed. 45 rats were randomized into three groups after 4 weeks and administered SSR (10 mL/kg daily by gavage,n = 15),losartan (6 mL/kg daily by gavage,n = 15) or saline treatment. The sham group underwent the same anesthesia and procedures consisting of manipulation of the perirenal fat and renal pedicles, except for the destruction of renal tissue. A group of 15 rats that received sham operation were also included in the study. After 8 weeks intervention,all animals were anaesthetized with sodium pentobarbital (40 mg/kg) administered intraperitoneally and euthanized by cervical dislocation. The remnant kidney tissues were collected for molecular examination. The animal procedures were approved by the Animal Experiment Ethics Committee of Shanghai University of Traditional Chinese Medicine in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Immunoprecipitation analysis
Kidney tissues were lysed on ice for 15 min in lysis buffer containing protease inhibitor. Approximately 500 μg of total protein was incubated overnight at 4 °C with anti-Smad3(Santa Cruz,USA) followed by precipitation with 70 μl of protein A/G-Plus-Agarose (Santa Cruz,USA) for 4 h at 4 °C.Non-specific IgG (Proteintech) was used as negative control. The precipitated complexes were washed in IP buffer and then, resuspended in 30 μl of 2× loading buffer and boiled for 5 min.
Western blot
The proteins were isolated from the frozen kidneys and the concentration was calculated by the Bradford method. Immunoblotting examination was performed as previously described [21]. In this study, the primary antibodies used were anti-Ecadherin (1:1000,Abcam,UK), anti-ColIII (11,000, Abcam, UK),anti-caspase1. (1:1000, Abcam,UK), anti-TGFβR1 (1:1000,Abcam,UK), anti-Sirt1 (11,000, Abcam, UK), anti-NLRP3 (1:1000, Abclonal, China), anti-IL-1β (11,000,Abclonal,China), anti-Acetylated-lysine (1:500,Santa Cruz,USA), anti-ASC (1:500, Santa Cruz, USA), anti-TGF-β1 (1500,Santa Cruz,USA), anti-Smad3 (1,1000,CST,USA), and anti-Gapdh (12,000, Proteintech, USA). Protein bands were visualized using the chemiluminescence system (Tanon) for the required time. Quantitative analysis was performed using ImageJ software.
Histology staining and evaluation
Paraffin-embedded kidneys were sectioned into 3 μm slices and subjected to Masson’s trichrome and periodic acid-Schiff (PAS) staining according to the standard protocol. For PAS-stained sections,the severity of glomerular injury was assessed in 50 glomeruli using the glomerulosclerosis (GS) score from 0 to 4 and the mean score was calculated [23]. The severity of tubulointerstitial fibrosis was calculated by the Masson’s trichrome-positive areas in 4 randomly selected fields per section of kidney using Image-Pro Plus 6.0.
Immunohistochemical (IHC) and immunofluorescence (IF) staining
IHC staining was performed as described previously [21]. In this study, the primary antibodies used were anti-Ecadherin (1200,Abcam) and anti-Sirt1 (1200, Abcam). Analysis of semi-quantification was performed in 4 randomly selected fields per section of kidney using Image-Pro Plus 6.0.For immunofluorescence staining,antigen was retrieved by microwave-EDTA buffer antigen retrieval method and blocked with 3% BSA and 0.1% Triton-100 after quenching autofluorescence. The sections were incubated with anti-αSMA (1200,Abcam,UK) and anti-F4/80(1200,Servicebio, China) overnight at 4 °C.The following secondary antibodies were used: FITC-labeled goat anti-rabbit IgG (Beyotime,China) and Cy3-labeled goat anti-mouse IgG (Beyotime,China). Positive staining was observed by fluorescence microscopy (Nikon Eclipse 80i, Japan) at 400 × magnification.
Statistical analysis
All results were expressed as mean ± standard deviation (SD) and analyzed by one-way analysis of variance (ANOVA) with LSD-t’s multiple comparisons, using SPSS version 18.0 software. P < 0.05 was considered statistically significant.
Results
SSR attenuated renal injury and fibrosis in 5/6(A/I) model
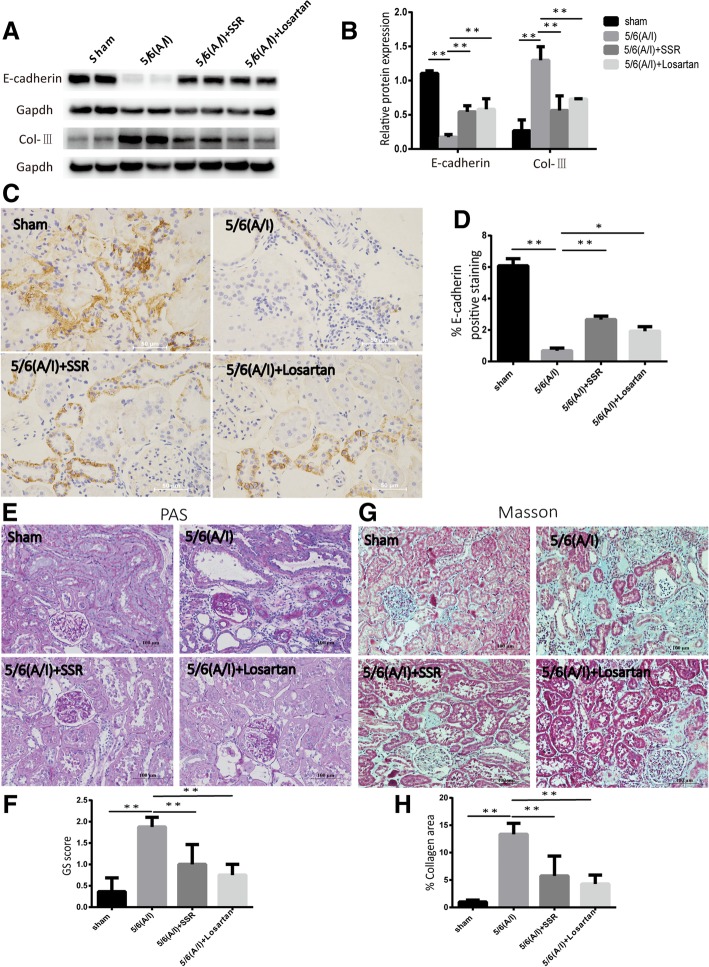
In previous studies,we confirmed SSR significantly attenuated the expression of Collagen-I (Col-I) and fibronectin (FN) proteins,two major ECM proteins, as well as α-smooth muscle actin (α-SMA) protein,the hallmark of fibroblast activation [21]. Based on the previous results, the study aimed to further assesse the expression of Collagen-III (Col-III) and E-cadherin proteins. Immunoblotting examination showed the expression of Col-III and loss of the epithelial cell marker E-cadherin were increased in the remnant kidneys (Fig. 1(a) and (b)).The IHC analysis of E-cadherin expression showed the same pattern as that of immunoblotting in 5/6(A/I) group (Fig. 1(c) and (d)). Conversely,SSR treatment markedly increased E-cadherin expression and decreased Col-III expression at protein levels in 5/6 (A/I) rats (Fig. 1(a),(b),(c) and(d)).
Fig. 1.
SSR attenuated renal injury and fibrosis in 5/6(A/I) model. (a) Immunoblot analysis of E-cadherin and Col-IIIexpression. (b) Western blot quantification of E-cadherin and Col-III levels.(n = 6) (c) Representative images of E-cadherin expression detected by IHC. 400 × magnification. (d) Quantitative analysis of E-cadherin positive area(n = 4) (e) Representative photomicrographs of PAS staining. 200 × magnification. (f) The severity of glomerular injury was assessed using the glomerulosclerosis (GS) score (g) Assessment of interstitial fibrosis by Masson’s trichrome staining.200 × magnification. (h) Semiquantitative result of collagen area(n = 4). Values are mean ± SD. *P < 0.05, **P < 0.01
Consistent with above observations, histopathological examination confirmed SSR showed significant reduction in interstitial fibrosis and renal injury when compared with model group (Fig. 1(e),(f),(g)and1(h)).SSR treatment for 8 weeks displayed less tubular injury, tubulointerstitial inflammation,and fibrosis after 5/6(A/I) operation,verifying a protective effect for SSR in renal injury (Fig. 1(e),(f), (g)and1(h)).
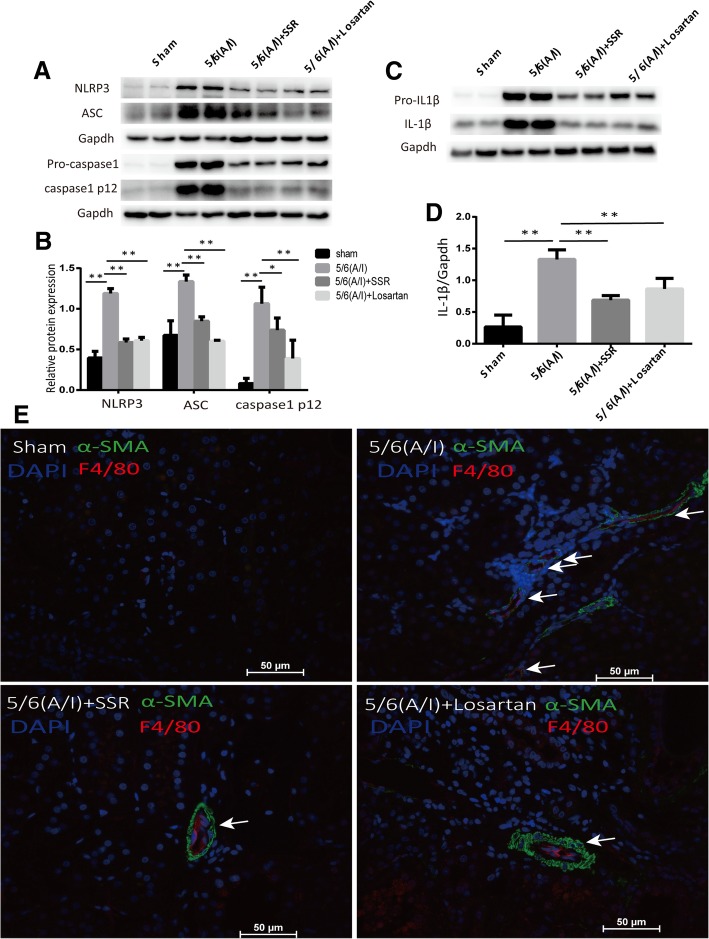
SSR inhibited NLRP3 inflammasome activation and IL-1β secretion in 5/6(A/I) rats.
Inflammation plays an important role in the development of renal fibrosis [24]. Renal NLRP3 inflammasome activation characterized by the elevation of NLRP3,ASC and Caspase-1 activation at protein levels was observed in the remnant kidneys with CRF,which was restored by SSR (Fig. 2(a) and2(b)).Results of inflammasome activation led to secretion of proinflammatory cytokine IL-1β(Fig. 2(c) and (d)).Consistent with above results, SSR decreased protein levels of mature IL-1β compared with 5/6(A/I) operation group,which was determined by immunoblotting for the 17-KD cytokine. (Fig. 2(c) and (d)).
Fig. 2.
SSR inhibited NLRP3 inflammasome activation and IL-1βsecretion in 5/6(A/I) rats. (a) The protein expression of NLRP3,ASC, Pro-caspase1 and Caspase-1 p12 was determined by immunoblotting. (b) Western blot quantification of NLRP3, ASC, and Caspase-1 p12 levels. (n = 6) (c) The protein levels of Pro-IL1β and IL1βwere determined by immunoblotting. (d) The ratio of IL-1βto GAPDH protein was determined. (n = 6) (e) Representative images of IF staining for α-SMA and F4/80. Original magnification,× 400. Values are mean ± SD. *P < 0.05, **P < 0.01
To determine whether the tubular injury and renal interstitial fibrosis are associated with inflammatory response,we mainly probed the position of F4/80 positive macrophages and myofibroblast marker α-SMA using immunofluorescence double staining. Our results presented the positional correlation between F4/80 and α-SMA (Fig. 2e). In addition,the model group reciving 5/6(A/I) operation displayed increased expression of F4/80 and α-SMA proteins compared with sham-operated group. Conversely,SSR dramatically ameliorated these expression of F4/80 and α-SMA (Fig. 2e). Taken together,our results revealed SSR effectively inhibited NLRP3/ASC/Caspase-1/IL-1β cascade and decreased inflammatory infiltration in the remnant kidneys,whcih may be associated with alleviated renal injury and interstitial fibrosis.
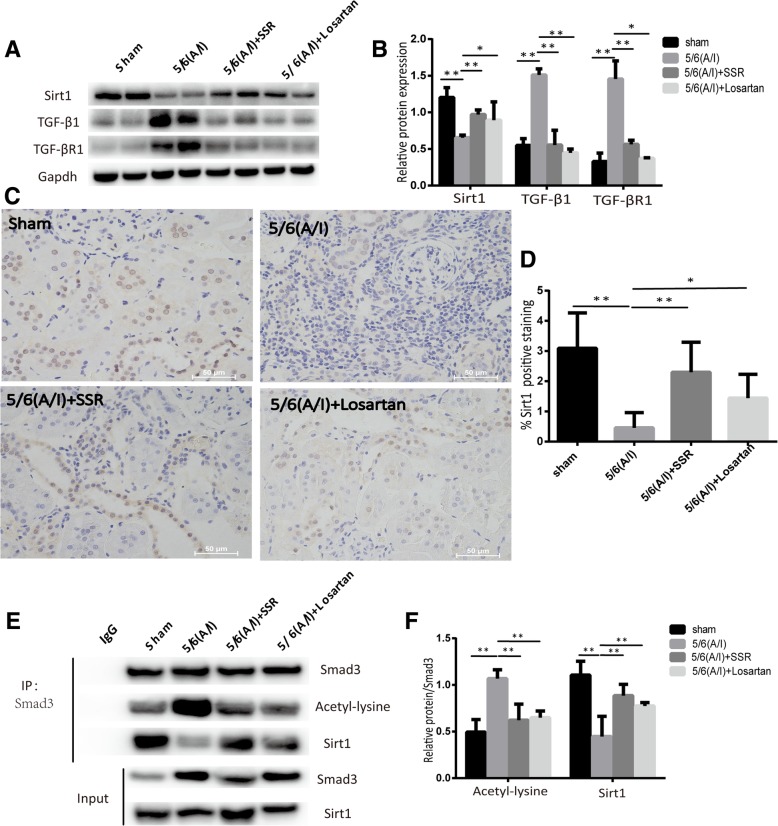
SSR reduced Smad3 acetylation in 5/6(A/I) rats by regulating Sirt1/Smad3 deacetylation pathway
Previous reports have shown that Sirt1 could mediate a variety of cellular responses by deacetylating lysine residues such as p53, NF-κB, and PGC-1α [25–27]. In this study, we first measured the expression of Sirt1 protein. As shown in Fig. 3(a), (b), (c)and (d),compared wtih sham-operated group,immunoblotting and IHC staining showed that injury to kidney decreased the expression of Sirt1 protein,whereas treatment with SSR markedly preserved Sirt1 expression (Fig. 3(a), (b), (c)and (d)).IHC staining confirmed Sirt1 is mainly located in the nucleus (Fig. 3c).Moreover,immunoblotting examination showed expression of typeITGF-βR (TGF-βR1) and TGF-β1 proteins was increased in the 5/6 (A/I) model group as compared to that in the sham-operated group (Fig. 3(a) and (b)).Meanwhile, SSR suppressed the expression of TGF-β1 and TGF-βR1 proteins compared with model group.
Fig. 3.
SSR reduced Smad3 acetylation in 5/6(A/I) rats by regulating Sirt1/Smad3 deacetylation pathway. (a) The protein levels of Sirt1,TGF-β1 and TGF-βR1 were determined by immunoblotting. (b) The ratio of Sirt1,TGF-β1 and TGF-βR1 to GAPDH protein was calculated. (n = 6) (c) Representative images of IHC staining for Sirt1. 400 × magnification. (d) Quantitative analysis of Sirt1 positive area(n = 4)(e) Immunoprecipitation and western blot analysis of Sirt1 and Smad3 acetylation in each group. (f) The ratio of Sirt1 and acetylated Smad3 to precipitated Smad3 protein was calculated. (n = 4) Values are mean ± SD. *P < 0.05, **P < 0.01
In addition, to assess the levels of acetylated Smad3,Smad3 was immunoprecipitated and the acetyl group was determined by an anti-acetyl antibody. As shown in Fig. 3(e) and (f),we observed that the acetylation levels of Smad3 increased in 5/6(A/I) rats and decreased in the rats treated with SSR.Furthermore,co-immunoprecipitation and Western blot studies revealed a direct interaction between Smad3 and Sirt1.The Smad3-Sirt1 binding was reduced in model group, which could be associated with increased levels of acetylated Smad3 (Fig. 3(e) and (f)).Conversely,SSR significantly increased the binding of Sirt1 and Smad3 and reduced acetylation levels of Smad3 (Fig. 3(e) and (f)).These data strongly suggest that renal protective effect of SSR may be associated with Sirt1/Smad3 deacetylation pathway.
Discussion
In the present study, we found that SSR could significantly suppress progressive activation of NLRP3/ASC/Caspase-1/IL-1β cascade and up-regulate Sirt1/Smad3 deacetylation pathway in the 5/6 (A/I) rat model. These changes could inhibit the expression of Col-III,the ECM protein,as well as E-cadherin,the epithelial cell marker. Together, these results identified that SSR significantly attenuated renal injury and fibrosis in the CRF model. In addition, our results showed that losartan, an angiotensin receptor blocker, seemed to be more effective than SSR in attenuating renal injury and fibrosis, inhibiting activation of NLRP3 complex and down-regulating the expression of TGF-β1 and TGF-βR1 proteins. Losartan may significantly reduce the increase of angiotensin II (Ang-II) induced by ischemia because 5/6(A/I) model is a typical intrarenal ischemia model [22, 28]. However, the specific mechanisms need further research.
Nonmicrobial (sterile) inflammatory response is an important characteristic of many CKDs [29].NLRP3 inflammasome is known to be activated by a variety of nonmicrobial signals,and therefore, NLRP3-dependent inflammation could be an attractive candidate as a mediator of the inflammatory component observed in CKD [8]. Previous studies reported NLRP3 complex was involved in the progression of kidney diseases in UUO model [9],ischemia/reperfusion injury (IRI) [30], and diabetic nephropathy [31] etc. and traditional Chinese herbs had obvious inhibition on it. Ren et al. [32]. reported Coptidis Rhizoma could attenuate early renal injury in obesity-related glomerulopathy (ORG) rats and the mechanisms were associated with the inhibition of NLRP3 inflammasome. Chang et al. [33]. demonstrated that rhein suppressed the NLRP3 inflammasome and macrophage activation in urate crystal-induced gouty inflammation. In addition, many active ingredients of SSR, such as magnesium lithospermate B from Salvia miltiorrhiza bunge, ferulic acid from Angelica sinensis,icariin from Epimedium, have shown anti-inflammatory effects in kidney diseases [34–36]. The activation of NF-κB signaling pathway stimulated renal inflammation in different models [37, 38]. Meanwhile,many reports revealed that NF-κB signaling pathway played a prime role in NLRP3 inflammasome activation [39, 40]. We have investigated the activity of NF-κB by phosphorylation of p65, its translocation to nucleus. Our results showed that SSR significantly reduced the nuclear translocation of p65 in 5/6(A/I) model [20]. These results prompted us to further study the possible role of NLRP3 inflammasome in 5/6(A/I) model, as well as the mechanisms of SSR attenuating renal fibrosis. Our results showed progressive activation of NLRP3/ASC/Caspase-1 cascade in CRF rat model,which led to the secretion of mature IL-1β pro-inflammatory factor. Inflammation initiates and sustains renal tubulointerstitial fibrosis [41],therefore,we probed colocalization of F4/80,a representative hallmark of macrophages,and α-SMA,a marker of fibroblast activation using immunofluorescence double staining. Our data showed that the increased F4/80 and α-SMA co-localized in the renal tubular areas. Conversely, activation of NLRP3 inflammasome analysed by immunoblotting and image analysis of IF staining showed significant down-regulation in treatment with SSR,verifying that one of the mechanisms by which SSR attenuates renal injury and delays the progression of renal interstitial fibrosis could be to inhibit inflammatory response induced by activation of NLRP3 complex.
Chronic inflammation is integrally associated with progressive CKD, as cytokines (TGF-β1, IL-18, IL-1β,etc.) produced by infiltrating leukocytes such as macrophages contribute significantly to renal injury and fibrosis [42].TGF-β1 promotes tissue fibrosis through the Smad3 pathway. While phosphorylation regulates Smad3 function, acetylation/de-acetylation of specific lysine residues in Smad3 has been shown to regulate TGF-β1 signaling by non-canonical signaling pathways [15]. In vitro,previous studies confirmed Smad3 could be acetylated in response to TGF-β1 stimulation [43, 44]. Recently, the lysine deacetylase sirtuin 1 (SIRT1) has been shown to exert renal protective effects [45]. Huang et al. [46]. reported that resveratrol, an activator of the Sirt1, ameliorated renal fibrosis by inhibiting the TGF-β1/Smad3 pathway. Here, we investigated whether pharmacological activation of Sirt1 would induce renal protection in 5/6(A/I) model. In this study, we showed the binding of Sirt1 with Samd3 and inhibition of this binding was associated with increased levels of acetylated Smad3. SSR down-regulated the acetylation levels of Smad3 by activating Sirt1 deacetylation pathway. These data highlight that renal Sirt1/Smad3 deacetylation pathway could be an antifibrotic factor and a potential therapeutic strategy for CKD.
Conclusions
In summary, we demonstrated that SSR could mediate renal protection by inhibiting NLRP3/ASC/caspase-1/IL-1β pathway and, furthermore,strengthen the antifibrotic effects by up-regulating Sirt1/Smad3 deacetylation pathway in 5/6th (A/I) model rats with CRF.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China General Projects (81573946), Scientific Research Foundation of Science and Technology Commission of Shanghai Municipal Government (16401931700), Shanghai Three Year Project of Traditional Chinese Medicine(ZY(2018-2020)-FWT X-7005). The funding bodies did not participate in the design of the study; collection, analysis, and interpretation of data; and writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 5/6 (A/I)
5/6ablation/ infarction
- Ang-II
AngiotensinII
- BUN
Blood urea nitrogen
- CKD
Chronic kidney disease
- Col-I
Collagen-I
- Col-III
Collagen-III
- CRF
Chronic renal failure
- ECM
Extracellular matrix
- eGFRs
Evaluated glomerular filtration rates
- FN
Fibronectin
- GS
Glomerulosclerosis
- IF
Immunofluorescence
- IHC
Immunohistochemical
- IL
Interleukin
- IRI
Ischemia/reperfusion
- ORG
Obesity-related glomerulopathy
- PAS
Periodic acid-Schiff
- Scr
Serum creatinine
- SD
Sprague-Dawley
- Sirt1
Sirtuin1
- SSR
ShenShuai IIRecipe
- TCM
Traditional Chinese medicine
- TGFβ-1
Transforming growth factorβ-1
- TGF-βR1
TypeITGF-βR
- α-SMA
α-smooth muscle actin
Authors’ contributions
CW designed the study and participated in data analysis and interpretation.MW and LYY finalized the experimental work, interpreted the results and prepared Figs.MW wrote the paper, CW and JY edited and revised. All authors read and approved the final version of manuscript.
Ethics approval and consent to participate
The animal procedures were approved by the Animal Experiment Ethics Committee of Shanghai University of Traditional Chinese Medicine in accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Meng Wang, Email: twsa927101@163.com.
Liuyi Yang, Email: 519413786@qq.com.
Jing Yang, Email: yangcerry2@163.com.
Chen Wang, Phone: (0086)13641836499, Email: chenwang42@126.com.
References
- 1.Mutsaers HA, Olinga P. Editorial: organ fibrosis: triggers, pathways, and cellular plasticity. Front Med. 2016;3:55. doi: 10.3389/fmed.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, Islam N, Bravo Rodolfo F, Aleckovic-Halilovic M, Zou H, Zhang LX, Gouda Z, Tchokhonelidze I, Abraham G, Mahdavi-Mazdeh M, Gallieni M, Codreanu I, Togtokh A, Sharma SK, Koirala P, Uprety S, Ulasi I, Remuzzi G. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4:e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 3.Eddy AA. Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl. 2014;4:2–8. doi: 10.1038/kisup.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djudjaj S, Boor P. Cellular and molecular mechanisms of kidney fibrosis. Mol Asp Med. 2018:1–21. [DOI] [PubMed]
- 5.Li ZL, Lv LL, Tang TT, Wang B, Feng Y, Zhou LT, Cao JY, Tang RN, Wu M, Liu H, Crowley SD, Liu BC. HIF-1αinducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388–404. doi: 10.1016/j.kint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Ting JY, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. The NLR gene family: a standard nomenclature. Immunity. 2018;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 InflammasomeActivation. Trends Biochem Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, Liu X, Huang YJ, Cai H, Zhan XY, Han QY, Wang H, Chen Y, Li HY, Li AL, Zhang XM, Zhou T, Li T. NLRP3 phosphorylation is an essential priming event for Inflammasome activation. Mol Cell. 2017;68(1):185–197. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Vilaysane A, Chun J, Seamone ME, Wang WJ, Chin R, Hirota S, Li Y. ClarkSA,Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang Y, Yasinta M, Hu C, Zhao M, Ding G, Bai M, Yang LY, Ni JJ, Wang R, Jia ZJ, Huang SG, Zhang AH. Mitochondrial dysfunction confers albumin-induced nlrp3 inflammasome activation and renal tubular injury. Am J Physiol Renal Physiol. 2015;308(8):F857–F866. doi: 10.1152/ajprenal.00203.2014. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig-Portugall Isis, Bartok Eva, Dhana Ermanila, Evers Beatrix D.G., Primiano Michael J., Hall J. Perry, Franklin Bernardo S., Knolle Percy A., Hornung Veit, Hartmann Gunther, Boor Peter, Latz Eicke, Kurts Christian. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney International. 2016;90(3):525–539. doi: 10.1016/j.kint.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 13.Roberts AB, Russo A, Felici A, Flanders KC. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann N Y Acad Sci. 2003;995:1–10. doi: 10.1111/j.1749-6632.2003.tb03205.x. [DOI] [PubMed] [Google Scholar]
- 14.Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006;17:19–27. doi: 10.1016/j.cytogfr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Xl Q, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–1071. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao CM, Haase VH. Sirtuins and their relevance to the kidney. J Am Soc Nephrol. 2010;21:1620–1627. doi: 10.1681/ASN.2010010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Wj WYY, Zhang MZ, You L, Davis LS, Fan H, Yang HC, Fogo AB, Zent R, Harris RC, Breyer MD, Hao CM. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120:1056–1068. doi: 10.1172/JCI41563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JW, Liu XF, Chen HZ, Sun ZY, Chen H, Wang L, Sun XH, Li XQ. Multi-targeting chemoprevention of Chinese herb formula Yanghe Huayan decoction on experimentally induced mammary tumorigenesis. BMC Complement Altern Med. 2019;19:48–62. doi: 10.1186/s12906-019-2456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu YY, He Z, Zhou Y, Yang J, Wang C. Effects of the meliorated renal FailureDecoction combined with Western medicine on renal function and renal perfusion in primary chronic kidney disease 3 or 4 stage patients. J Tradit ChinMed. 2018;59(17):1480–1484. [Google Scholar]
- 20.Yang J, Zhu TT, Wang C. Effects of Shenshuai IIDecoction on NF-κB /TNF-α signaling pathway expressions in renal tissues of chronic renal failure rats. Chin J Inform on TCM. 2018;25(8):58–62. [Google Scholar]
- 21.Wang Meng, Yang Jing, Zhou Yuan, Wang Chen. ShenShuai II Recipe Attenuates Apoptosis and Renal Fibrosis in Chronic Kidney Disease by Increasing Renal Blood Flow and Improving Oxygen Consumption. Evidence-Based Complementary and Alternative Medicine. 2018;2018:1–8. doi: 10.1155/2018/7602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng AH, Tang T, Singh P, Wang C, Satriano J, Thomson SC, Blantz RC. Regulation of oxygen utilization by angiotensin II in chronic kidney disease. Kidney Int. 2009;75:197–204. doi: 10.1038/ki.2008.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Han WZ, Zhang M, Fang WY, Zhai XR, Guan SF, Qu XK. Long-term renal sympathetic denervation ameliorates renal fibrosis and delays the onset of hypertension in spontaneously hypertensive rats. Am J Transl Res. 2018;10:4042–4053. [PMC free article] [PubMed] [Google Scholar]
- 24.Lv W, Booz GW, Wang YG, Fan F, Roman RJ. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76. doi: 10.1016/j.ejphar.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan F, Xie Q, Wu J, Bai Y, Mao B, Dong Y, Bi W, Ji G, Tao W, Wang Y, Yuan Z. MST1 promotes apoptosis through regulating Sirt1-dependent p53 deacetylation. J Biol Chem. 2011;286:6940–6945. doi: 10.1074/jbc.M110.182543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NFkappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amat R, Planavila A, Chen SL, Iglesias R, Giralt M, Villarroya F. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J Biol Chem. 2009;284:21872–21880. doi: 10.1074/jbc.M109.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.ASN.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- 29.Ricardo SD, van GH, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HJ, Lee DW, Ravichandran K, O-Keys D, Akcay A, Nguyen Q, He ZB, Jani A, Ljubanovic D, Edelstein CL. NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther. 2013;346:465–472. doi: 10.1124/jpet.113.205732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K, Zhang J, Zhang W, Yang J, Li K, He Y. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45:932–943. doi: 10.1016/j.biocel.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Yl R, Dk W, Lu F, Zou X, Lj X, Wang KF, Huang WY, Su H, Zhang C, Gao Y, Dong H. Coptidis Rhizoma inhibits NLRP3 inflammasome activation and alleviates renal damage in early obesity-related glomerulopathy. Phytomedicine. 2018;49:52–65. doi: 10.1016/j.phymed.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Chang WC, Chu MT, Hsu CY, Wu YJ, Lee JY, Chen TJ, Chung WH, Chen DY, Hung SL. Rhein, an Anthraquinone drug, suppresses the NLRP3 Inflammasome and macrophage activation in urate crystal-induced gouty inflammation. Am J Chin Med. 2019;47:135–151. doi: 10.1142/S0192415X19500071. [DOI] [PubMed] [Google Scholar]
- 34.Park CH, Shin SH, Lee EK, Kim DH, Kim MJ, Roh SS, Yokozawa T, Chung H. Magnesium Lithospermate B from Salvia miltiorrhiza Bunge ameliorates aging-induced renal inflammation and senescence via NADPH oxidase-mediated reactive oxygen generation. Phytother Res. 2017;31:721–728. doi: 10.1002/ptr.5789. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury S, Ghosh S, Das A, Sil P. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front Pharmacol. 2019;10:1–24. doi: 10.3389/fphar.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen HA, Chen CM, Guan SS, Chiang CK, Wu CT, Liu SH. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine. 2019;59:1–7. doi: 10.1016/j.phymed.2018.09.179. [DOI] [PubMed] [Google Scholar]
- 37.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft F, Scheidereit C, Muller D. Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Ha I, Hwang C, Lee YJ, Kim J, Yang SH, Kim YS, Cao YA, Choi S, Park WY. Gene expression profiling of anti-GBM glomerulonephritis model: the role of NF-kappaB in immune complex kidney disease. Kidney Int. 2004;66:1826–1837. doi: 10.1111/j.1523-1755.2004.00956.x. [DOI] [PubMed] [Google Scholar]
- 39.Bauernfeind F, Horvath G, Stutz A, Alnemri E, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks B, Fitzgerald K, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamkanfi M, Dixit V. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Xl S, Zou FF, Xu TJ, Pan PH, Hu CP. Toll-like receptor-4 deficiency alleviates chronic intermittent hypoxia-induced renal injury, inflammation, and fibrosis. Sleep Breath. 2018:1–12. [DOI] [PubMed]
- 42.Wang WJ, Wang XY, Chun J, Vilaysane A, Clark S, French G, Bracey NA, Trpkov K, Bonni S, Duff HJ, Beck PL, Muruve DA. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J Immunol. 2013;190:1239–1249. doi: 10.4049/jimmunol.1201959. [DOI] [PubMed] [Google Scholar]
- 43.Simonsson M, Kanduri M, Grönroos E, Heldin CH, Ericsson J. The DNA bindingactivities of Smad2 and Smad3 are regulated by coactivator-mediated acetylation. J Biol Chem. 2006;281:39870–39880. doi: 10.1074/jbc.M607868200. [DOI] [PubMed] [Google Scholar]
- 44.Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku H, Fukamizu A, Onozaki K, Hayashi H. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26:500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- 45.Kitada Munehiro, Takeda Ai, Nagai Takako, Ito Hiroki, Kanasaki Keizo, Koya Daisuke. Dietary Restriction Ameliorates Diabetic Nephropathy through Anti-Inflammatory Effects and Regulation of the Autophagy via Restoration of Sirt1 in Diabetic Wistar Fatty (fa/fa) Rats: A Model of Type 2 Diabetes. Experimental Diabetes Research. 2011;2011:1–11. doi: 10.1155/2011/908185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang XZ, Wen DH, Zhang M, Xie QH, Ma L, Guan Y, Ren YH, Chen J, Hao CM. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J Cell Biochem. 2014;115:996–1005. doi: 10.1002/jcb.24748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.