Abstract
Adenophora triphylla (Thunb.) A.DC., a well-known herbaceous medicinal species, has been reported to protect against human obesity, cancer, and inflammation. Supplementary lighting is a practical strategy to improve crop quality, especially at a propagation stage. However, there has been no study available on the optimal supplementary light source for the commercial production of A. triphylla seedlings. In this study, plug seedlings were cultivated in a greenhouse for four weeks under an average daily light intensity of 490 μmol·m−2·s−1 PPFD coming from the sun and a supplemental lighting (16 h per day) at 120 μmol·m−2·s−1 PPFD provided by high pressure sodium (HPS), metal halide (MH), far-red (FR) light, white LED (red: green: blue = 2:4:3, LED-w), or mixed (red: green: blue = 4:1:4) LED (LED-mix). The results showed that LED-mix, with a higher percentage of red and blue light, substantially promoted seedling growth compared to other treatments by increasing stem diameter, biomass, specific leaf weight, and root to shoot ratio. The LED-mix also promoted accumulation of soluble sugar, starch, and chlorophyll in the tissue and increased contents of total phenols and flavonoids. Moreover, stomata density and pore area per leaf area under the LED-mix were remarkably greater than those under other treatments. Furthermore, the Western blot analysis revealed that the expression of photosynthetic protein, D1, was notably enhanced by the LED-mix as compared with other light sources. In addition, the LED-mix alleviated the oxidative damage of seedlings by improving enzymatic and nonenzymatic antioxidant systems. Collectively, these results suggest that the LED-mix was the optimal supplementary light source for the production of highest quality A. triphylla seedlings.
1. Introduction
Adenophora triphylla (Thunb.) A.DC. (Campanulaceae), also named as three-leaf lady bell or Japanese lady bell, is a perennial herb, which is mainly distributed in Korean Peninsula, China, Japan, and Russia (Far East and Eastern Siberia) [1, 2]. Besides its ornamental value, A. triphylla has been an important medicinal plant in oriental medicine for remedying whooping cough and chronic bronchitis in China [1]. In addition, this herb has also been used as a food source to prevent obesity in traditional Korean recipes [3]. In recent years, antitumor and antidiabetic activities have been reported in this species [4, 5]. Some previous studies attached the importance to extraction and identification of phytochemicals, such as lupenone, daucosterol, and adenophoric acid methyl ester, and to the action of disease resistance [3–5]. However, few studies have focused on the effect of light on the growth and development of this plant species.
Light is the most important environmental factor affecting photosynthesis and thus yield because plant growth and yield depend on photosynthesis [6, 7]. Plant morphology, physiology, and biochemistry also vary largely with the light source [8–10]. Light spectrum influences photosynthetic process by adjusting stomatal development and movement [11], photosynthetic pigment level [12], and photosynthetic protein biosynthesis and activities of light-harvesting complexes (LHC), photosystem I (PSI), photosystem II (PSII), and cytochrome b6f (Cyt b6f) [13]. Monochromatic light, especially red and blue light, has been widely studied [14, 15] for their effects. For example, many studies proved that red light affects stem elongation, leaf extension, bud outgrowth, and photosynthetic apparatus [11, 16], while blue light influences leaf thickness, hypocotyl elongation, photosynthesis, biosynthesis and accumulation of secondary metabolites, and stomata development and opening [10, 17, 18]. However, monochromatic light is not sufficient for growth and development for some plant species, leading to lower biomass, abnormal leaf morphology, and reduced photosynthesis as compared with mixed light [18–21]. Therefore, broad-band or dichromatic light source may have to be employed as an artificial light source in some horticultural plant species and their beneficial effects and the molecular mechanism involved should be focused on and deeply studied in the future.
Supplementary lighting is an important horticultural practice and strategy to improve crop growth and to obtain all-year-round, high-yield, and superior-quality productions in greenhouses [19, 22]. In commercial productions, plants are usually provided with an additional lighting at an intensity between 100 and 200 μmol·m−2·s−1 photosynthetic photon flux density (PPFD) for up to 16 h per day (even 20 h in some regions) to maximize production [20, 23]. Source of supplementary light is, therefore, a key factor in determining the effect of this practice. Traditionally, high pressure sodium (HPS) and metal halide (MH) lamps belonging to high intensity discharge (HID) lamps have been the most commonly used artificial light sources for plant research and greenhouse horticulture [18, 24]. Moreover, supplementary far-red (FR) light has been proved to enhance plant biomass, stem length, leaf length, and leaf width [22, 25]. Recently, a new light source, light emitting diodes (LEDs), has gained widespread attention, since it provides a narrow special wavelength band and a high efficiency [12, 26, 27]. However, data are still scarce regarding the choice of light source and how the supplementary light source influences the growth, physiology, and biochemistry of plug seedlings, especially in plug seedlings of medicinal plants.
Different supplementary light sources may influence primary and secondary metabolism in different manners [20, 28]. Previous studies have shown that conventional HID lamps are more efficient in the improvement of metabolites than LEDs [24, 29, 30]. However, there are studies suggesting that LEDs are more advantageous over traditional light sources in increasing primary and secondary metabolites [31–33]. Therefore, more studies are required to provide more evidences to clear the ambiguity, notably in medicinal plants including A. triphylla.
In this study, it was hypothesized that mixed LEDs could be an alternative supplementary light source for stimulating growth and metabolism and, therefore, improving quality of A. triphylla seedlings as compared with conventional light sources. To test this hypothesis and accelerate the production of high quality A. triphylla seedlings for commercial exploitation, HPS, MH, FR, white LEDs, and mixed LEDs were employed as supplementary light sources during a rainy summer season. In the study, spectral characteristics of those light sources, growth characteristics of seedlings grown under those light sources, and accumulation of primary and secondary metabolites in seedlings were investigated. Moreover, to reveal how the light source drives plant growth and affects seeding quality, stomata properties, expression of photosynthetic proteins, and redox homeostasis were also examined. The data obtained may provide a theoretical and practical basis for improving growth and development, and also enhancing the medicinal value of A. triphylla seedlings by supplying an optimal light source. The data may also be helpful in guiding the growers for cultivation and management of A. triphylla and other medicinal plants.
2. Materials and Methods
2.1. Supplementary Light Sources and Cultivation Conditions
In this study, five different light sources (Figure 1), high pressure sodium (HPS, BLV Licht-Und Vakuumtechnik, Steinhöring, Germany), metal halide (MH, SunLumen Lighting Co. Ltd, Gyeongju, South Korea), far-red (FR, Philips Lighting, Amsterdam, The Netherlands), white (red: green: blue = 2:4:3) LED (LED-w, Victory Lighting Ltd, Seoul, South Korea), and mixed (red: green: blue = 4:1:4) LED (LED-mix, Custom made, SungKwang LED Co. Ltd, Incheon, South Korea), were tested. The supplementary light intensity was set uniformly at 120 μmol·m−2·s−1 PPFD with a 16 h photoperiod, while an average daily maximum light intensity coming from the sun was about 490 μmol·m−2·s−1 PPFD with a natural photoperiod of 14 h. Seedlings were cultivated in a glasshouse at an average day/night temperatures of 31.6°C/26.3°C (average daily maximum temperature is 34.5°C) and 88.1% relative humidity for four weeks. After cultivation, some plants were harvested for measurements of growth and physiological parameters, and others were frozen in liquid nitrogen immediately after harvest, and then were stored in a −80°C freezer for further analyses.
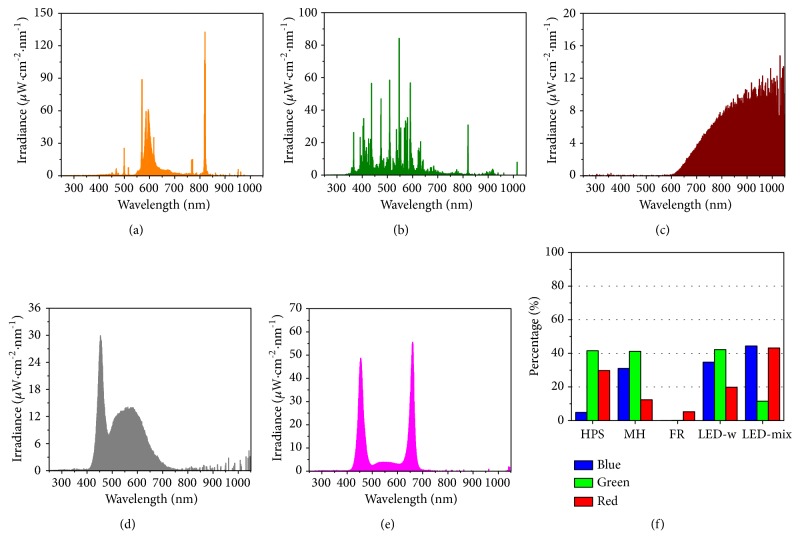
Figure 1.
The spectral distribution of high pressure sodium (HPS, (a)), metal halide (MH, (b)), far-red (FR, (c)), white (red: green: blue = 2:4:3) light-emitting diodes (LED-w, (d)), and mixed (red: green: blue = 4:1:4) light-emitting diodes (LED-mix, (e)), and percentages of blue, green, and red (f) light for each supplementary light source used in the study. Irradiance was measured by a traceable calibrated spectroradiometer (ILT950 NIST, International Light Technologies, Inc., Peabody, MA, USA) without sunlight.
2.2. Analysis of Stomata Using Scanning Electron Microscopic (SEM)
Sample preparation and SEM analysis of stomata were carried out following previously published method [28, 34]. In brief, excised leaves were fixed immediately in a 2.5% glutaraldehyde solution at 4°C overnight. Then samples were stained with 1% osmic acid (OsO4) at 4°C for 2 hours. Subsequently, they were dehydrated by a graded series of ethanol, followed by wash with 80% acetone. After fixation and staining, samples were washed carefully by a 0.1 M phosphate-buffered saline solution (PBS, pH 7.0). Finally, dried samples were gold coated, observed, and photographed by using a scanning electron microscope (JSM-6380, Jeol Ltd., Tokyo, Japan).
The stomata-related parameters were analyzed by using software (version 1.8.0, ImageJ, available online at https://imagej.nih.gov/ij/download.html) according to previous definitions [35, 36]. In brief, the length and width of the guard cell, and the length and width of the pore, were measured based on the definition of Sack [35]. The guard cell area was presented as the length of guard cell multiplied by the width of the guard cell pair, while the pore area was calculated as the pore length multiplied by two times of the pore width [37]. The stomata density and number of stomata per area were calculated as the stomata number divided by the measured area, where the stomata number was counted. The pore area per leaf area was presented as the total pore area divided by the recorded area, where the stomata number was counted. The stomatal aperture was calculated as the pore width divided by the pore length [36].
2.3. Measurements of Soluble Sugar, Starch, Soluble Proteins, Total Phenols, and Flavonoids
The contents of soluble sugar and starch were measured by the anthrone colorimetric method as described by Xue et al. [38]. Soluble proteins were extracted by a sodium phosphate buffer and then measured colorimetrically by the Bradford method based on previous publication [39]. Total phenols and flavonoids were extracted with 80% methanol. The contents of total phenols and flavonoids were estimated by the previously described methods by Manivannan et al. [40].
2.4. Localization of H2O2 by DAB Staining and Antioxidant Enzyme Activity Assays
Excised leaves were immersed in a 0.1% 3,3′-diaminobenzidine (DAB) solution containing 0.05% Tween 20 and 0.0175% H2O2, followed by a vacuum infiltration for 15 min and an incubation in dark condition for 2 hours. Then leaves were washed carefully three times with distilled water and then immersed in absolute ethanol. Finally, leaves were boiled in hot water until all leaves become white. The activities of sodium dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX) were determined according to the protocols described by Muneer et al. and Soundararajan et al. [34, 41], respectively.
2.5. Quantifications of Photosynthetic Components and Immunoblot Analysis
Chlorophyll content was determined by using a chlorophyll meter (SPAD-502 Plus, Konica Minolta Inc., Osaka, Japan). Chloroplast proteins were extracted based on the methods of Muneer [42, 43] and then separated by sodium dodecyl sulfate PAGE (SDS-PAGE). The intensity of protein bands was analyzed by using software (version 1.8.0, ImageJ, National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/download.html) [39]. For western blots of D1 protein, the protein was extracted by a 100 mM Tris-HCl buffer (pH 7.8) with 1 mM EDTA-Na2, 2% PVP, 1% triton X-100, and 0.07% β-mercaptoethanol according to a previously described protocol [28, 41]. Extracted protein (25 μg) was mixed well with a 240 mM Tris-HCl buffer (pH 6.8) containing 8% sodium dodecyl sulfate (SDS), 0.04% bromophenol blue, 40% glycerol, and 5% β-mercaptoethanol and then separated by the SDS-PAGE. Finally, the expression of D1 protein (anti-PsbA) was analyzed by the immunoblotting described by Muneer [42]. The extracted proteins for each sample were loaded on the gel using an equal soluble protein basis. And the contents of proteins were shown as a percentage relative to HPS.
2.6. Statistical Analysis
The experimental assays were performed with three times individual biological repeats and data were presented as the mean ± standard error (SE) of the mean. Data were statistically analyzed by one-way analysis of variance (ANOVA), followed by Duncan's multiple range test at p < 0.05, using a statistical analysis software (V. 9.12, Statistical Analysis System, Cary, NC, USA).
3. Results
3.1. Spectral Characteristics of Different Supplementary Light Sources
Different artificial light sources provide various spectral characteristics which may differ from the sunlight. In this study, the five supplementary light sources used clearly displayed diverse spectral features ranging from 250 to 1,050 nm (Figures 1(a)–1(e) and Table 1). Irradiance of the HPS was mainly in green band (41.6%) followed by red (29.7%) and blue (only 4.8%) bands (Figures 1(a) and 1(f), and Table 1). Similarly, the MH also showed a high percentage of irradiance in green band (41.1%). However, the MH had a higher proportion of blue (30.9%) and less red (12.3%) irradiance than the HPS. Moreover, irradiance of the FR was mainly concentrated in the wavelength range from700 to 1,050 nm (FR and IR) followed by 600 to 700 nm (red). The distribution of irradiance of the LED-w exhibited a broader band from 400 to 700 nm and had a distinct peak (at 453 nm) in blue. In contrast to the LED-w, the LED-mix possessed a narrow spectrum with two peaks at 454 and 660 nm, and irradiance was mainly distributed in blue (44.4%) and red (43.1%) bands.
Table 1.
The irradiance distributions of the different supplementary light sources used in this study.
| Treatment | Irradiance (μW·cm−2) | |||||
| UV | Blue | Green | Red | FR | IR | |
| (250-400 nm) | (401-500 nm) | (501-600 nm) | (601-700 nm) | (701-800 nm) | (801-1050 nm) | |
|
| ||||||
| HPS | 16.7 | 145.0 | 1,258.5 | 900.4 | 151.2 | 557.0 |
| MH | 244.4 | 925.5 | 1,231.5 | 369.2 | 83.5 | 138.6 |
| FR | 2.1 | 0.7 | 1.5 | 155.0 | 563.3 | 2,245.6 |
| LED-w | 15.8 | 1,053.0 | 1,279.3 | 599.3 | 39.2 | 48.2 |
| LED-mix | 10.4 | 1,309.5 | 341.0 | 1,273.4 | 11.1 | 6.9 |
HPS: high pressure sodium; MH: metal halide; FR: far-red; LED-w: white LED (red: green: blue = 2:4:3); LED-mix: mixed (red: green: blue = 4:1:4) LED; UV: ultraviolet; and IR: infrared radiation.
3.2. Growth, Development, and Morphology
In this study, growth and morphological characteristics of the seedlings were greatly influenced by supplementary light source (Figure 2 and Table 2). Stem diameter in the LED-mix was 3.8±0.2 mm, remarkably greater than that in the other treatments except the MH. Biomass, including total dry weight and shoot dry weight, showed a similar pattern that biomass in the LED-mix, LED-w, and MH was greater than that in the HPS and FR. More importantly, seedlings in the LED-mix presented strength to hold medium and to form a bigger root ball (Figure 2). Meanwhile, seedlings in the LED-mix had a significantly greater specific leaf weight (2.20±0.08 × 10−2 g·cm−2) than that in the HPS, MH, and FR (p < 0.05). Total seedling length showed no differences among treatments except the FR. The root to shoot ratio in the LED-mix was the greatest (0.42±0.05) while there were no differences in number of leaves among the treatments (p = 0.06 > 0.05).
Figure 2.
Morphological characteristics of A. triphylla seedlings grown with different supplementary light sources: HPS, high pressure sodium; MH, metal halide; FR, far-red; LED-w, white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes.
Table 2.
Growth characteristics of A. triphylla seedling grown under different supplementary light sources.
| Treatment | Stem diameter (mm) | Total length (mm) | Total DW (g) | Shoot DW (g) | Root DW (g) | SLW b (10−2 g·cm−2) | No. of leaves | Root to shoot ratio |
|
| ||||||||
| HPS | 3.0 ± 0.2 b | 207.9 ± 5.7 a | 0.19 ± 0.02 b | 0.15 ± 0.01 b | 0.04 ± 0.01 c | 1.95 ± 0.06 b | 4.8 ± 0.4 a | 0.30 ± 0.04 b |
| MH | 3.4 ± 0.2 ab | 211.3 ± 7.7 a | 0.31 ± 0.03 a | 0.24 ± 0.02 a | 0.07 ± 0.01 ab | 1.94 ± 0.09 b | 6.6 ± 0.6 a | 0.30 ± 0.04 b |
| FR | 2.2 ± 0.1 c | 172.9 ± 8.5 b | 0.11 ± 0.01 c | 0.09 ± 0.01 c | 0.02 ± 0.00 d | 1.53 ± 0.09 c | 4.7 ± 0.5 a | 0.21 ± 0.03 b |
| LED-w | 3.0 ± 0.1 b | 216.5 ± 10.6 a | 0.28 ± 0.03 a | 0.24 ± 0.02 a | 0.05 ± 0.01 bc | 2.21 ± 0.09 a | 5.6 ± 0.7 a | 0.22 ± 0.02 b |
| LED-mix | 3.8 ± 0.2 a | 199.8 ± 5.3 a | 0.30 ± 0.03 a | 0.22 ± 0.02 a | 0.08 ± 0.01 a | 2.20 ± 0.08 a | 6.0 ± 0.4 a | 0.42 ± 0.05 a |
DW: dry weight; SLW: specific leaf weight; HPS: high pressure sodium; MH: metal halide; FR: far-red; LED-w: white (red: green: blue = 2:4:3) LED; and LED-mix: mixed (red: green: blue =4: 1: 4) LED. Data are presented as the mean ± standard error of mean (n = 10). Different letters (a, b, and c) indicate significant differences (p < 0.05) among treatments.
3.3. Anatomical Feature of Stomata
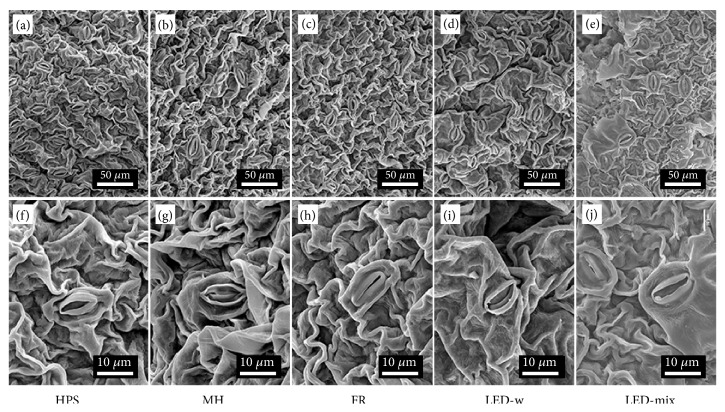
The stomatal characteristics were influenced by supplementary light source (Figure 3 and Table 3). The highest stomata density was found in the LED-mix (664.1±33.4 mm−2) followed by the HPS and LED-w. Stomatal density in the FR and MH is considerably lower than the other treatments. Adversely, the aperture of stomata showed a contrary feature except in the FR. The smallest aperture was found in the LED-mix, only 0.15±0.03, while the MH had the greatest aperture (0.26±0.02) followed by the LED-w and HPS. In addition, the pore length and the guard cell area in the MH were remarkably greater than those in other treatments. More importantly, the pore area per leaf area in the LED-w, LED-mix, and HPS was markedly greater than in the MH and FR. However, there was no difference in the guard cell length, guard cell width, pore width, and pore area among the treatments.
Figure 3.
Stomatal development in A. triphylla leaves after four weeks of cultivation under different supplementary light sources: HPS, high pressure sodium ((a) and (f)); MH, metal halide ((b) and (g)); FR, far-red ((c) and (h)); LED-w, white (red: green: blue = 2:4:3) light-emitting diodes ((d) and (i)); and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes ((e) and (j)). Bar, 10 ((f)–(j)) or 50 ((a)–(e)) μm.
Table 3.
Stomata characteristics in A. triphylla leaves after four weeks of cultivation under different supplementary light sources.
| Treatment | SD (mm−2) | GCL (μm) | GCW (μm) | PL (μm) | PW (μm) | GCA (μm2) | PA (μm2) | PAPLA (1000 μm2·mm−2) | Aperture |
|
| |||||||||
| HPS | 433.9 ± 21.2 b | 11.9 ± 1.2 a | 2.6 ± 0.6 a | 15.5 ± 0.4 b | 3.8 ± 0.2 a | 71.5 ± 1.3 c | 28.6 ± 8.7 a | 12.8 ± 4.5 a | 0.22 ± 0.03 ab |
| MH | 121.1 ± 31.7 c | 11.5 ± 3.2 a | 2.9 ± 0.6 a | 21.0 ± 0.3 a | 4.1 ± 0.1 a | 116.9 ± 8.9 a | 29.8 ± 12.1 a | 4.2 ± 2.0 b | 0.26 ± 0.02 a |
| FR | 98.8 ± 20.5 c | 12.5 ± 1.0 a | 2.3 ± 0.2 a | 17.3 ± 1.5 b | 3.7 ± 0.2 a | 80.1 ± 4.6 bc | 25.6 ± 2.0 a | 2.6 ± 0.7 b | 0.19 ± 0.03 ab |
| LED-w | 414.2 ± 10.1 b | 13.5 ± 0.2 a | 3.4 ± 0.4 a | 17.5 ± 0.3 b | 4.0 ± 0.3 a | 80.7 ± 4.1 bc | 38.4 ± 5.9 a | 15.8 ± 2.9 a | 0.25 ± 0.03 a |
| LED-mix | 664.1 ± 33.4 a | 13.0 ± 1.1 a | 1.9 ± 0.2 a | 17.3 ± 0.5 b | 4.0 ± 0.1 a | 98.6 ± 3.5 ab | 20.9 ± 2.2 a | 13.8 ± 1.0 a | 0.15 ± 0.03 b |
SD: stomata density; GCL: guard cell length; GCW: guard cell width; PL: pore length; PW: pore width; GCA: guard cell area; PA: pore area; and PAPLA: pore area per leaf area (1000 μm2·mm−2). HPS: high pressure sodium; MH: metal halide; FR: far-red; LED-w: white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix: mixed (red: green: blue = 4:1:4) light-emitting diodes. Different letters (a, b, and c) indicate significant differences (p < 0.05) among treatments.
3.4. Estimation of Photosynthetic Components
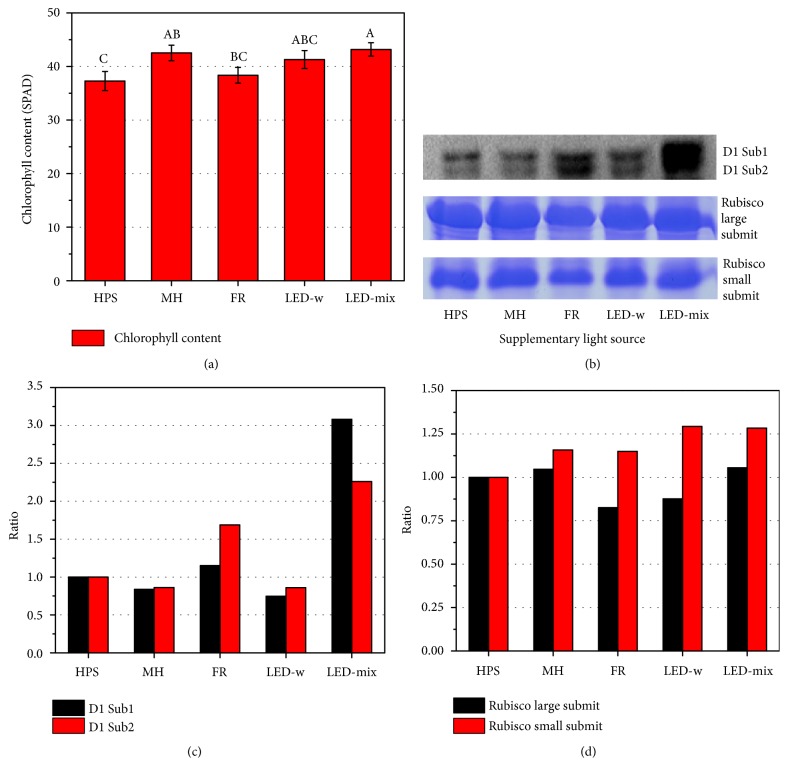
The data showed that supplemental light source impacted the chlorophyll content in leaves (Figure 4(a)). The greatest chlorophyll content (43.18±1.25 SPAD) was obtained in the LED-mix followed by the order of MH, LED-mix, FR, and HPS (Figure 4(a)). The chloroplast protein profile showed about 30 apparent proteins, with the molecular mass ranging from 10 to 175 kDa (Figure S1). They were ordered from the largest to the smallest based on their molecular masses and presented by their intensities (Figures S1B-G). As compared with the HPS, expression of most proteins in the MH (90%) and LED-mix (80%) was maintained at a higher level. Meanwhile, there were only 10% proteins in the FR and 50% in the LED-w higher than in the HPS.
Figure 4.
Effect of supplementary light source on chlorophyll content (a), expression (b), and relative contents of D1 protein (c) and Rubisco (d) in leaves of A. triphylla seedlings. D1 protein was analyzed by the Western blot/immunoblots analyses, while Rubisco was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The extracted proteins for each sample were loaded on the gel using an equal soluble protein basis. The contents of proteins were shown as a percentage relative to HPS. HPS, high pressure sodium; MH, metal halide; FR, far-red; LED-w, white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes.
The Western blot analysis revealed that the expression of D1 protein was higher in the LED-mix followed by the FR and HPS and was relatively lower in the LED-w or MH (Figure 4(b)). In detail, the expression of Sub1 and Sub2 in the LED-mix was 3.08 and 2.26 times as high as that in the HPS (Figure 4(c)), respectively. In the LED-w, the expression of Sub1 and Sub2 showed the lowest values, which were 0.75 and 0.86 times, respectively, the amount in the HPS (Figure 4(c)). The SDS-PAGE analysis showed that the expression of Rubisco in LED-mix was improved and Rubisco large submit and small submit were 1.06 and 1.28 times as high as that in HPS, respectively.
3.5. Contents of Soluble Sugar, Starch, and Soluble Proteins
The supplemental light source had influence on primary metabolites. As shown in Table 4, soluble sugar content in the LED-mix was the greatest (2.31±0.18%), which was 1.34 and 1.19 folds greater than that in the FR and MH, respectively. Meanwhile, content of starch in the LED-mix (1.13±0.04%) was greater than that in other treatments. However, there were no obvious differences in content of soluble proteins.
Table 4.
Contents of soluble sugar, starch, and soluble protein in A. triphylla leaves as affected by supplementary light source.
| Treatment | Soluble sugar (% of FW) | Starch (% of FW) | Soluble protein (% of FW) |
|
| |||
| HPS | 2.16 ± 0.05 ab | 1.01 ± 0.04 b | 0.87 ± 0.07 a |
| MH | 1.94 ± 0.04 ab | 1.04 ± 0.02 ab | 0.80 ± 0.06 a |
| FR | 1.73 ± 0.20 b | 1.00 ± 0.01 b | 0.85 ± 0.05 a |
| LED-w | 2.27 ± 0.09 a | 1.10 ± 0.02 ab | 0.84 ± 0.04 a |
| LED-mix | 2.31 ± 0.18 a | 1.13 ± 0.04 a | 0.83 ± 0.02 a |
FW: fresh weight; HPS: high pressure sodium; MH: metal halide; FR: far-red; LED-w: white (red: green: blue = 2:4:3) LED; and LED-mix: mixed (red: green: blue = 4:1:4) LED. Data are presented as the mean ± standard error of mean (n = 9). Different letters (a and b) indicate significant differences (p < 0.05) among treatments.
3.6. Contents of Total Phenols and Flavonoids
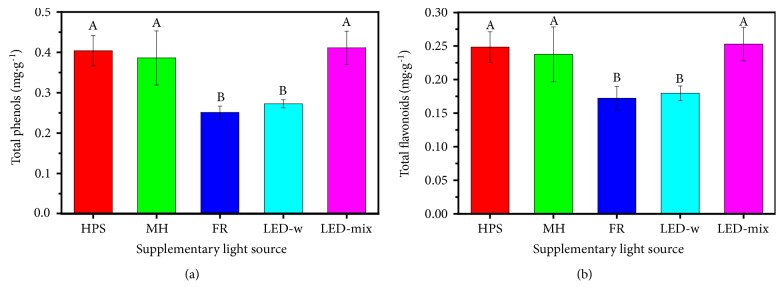
The supplementary light source not only affected the primary metabolism, but also influenced the secondary metabolism. In this study, the greatest content of total phenols (0.41±0.04 mg·g−1) was found in the LED-mix followed by the HPS and MH and was significantly greater than that in the LED-w and FR (Figure 5(a)) (p < 0.05). Similarly, total flavonoid content showed a similar tendency. Content of total flavonoid in the LED-mix was 0.25±0.03 mg·g−1, which was greater than that in the HPS, MH, LED-w, and FR (Figure 5(b)).
Figure 5.
Contents of total phenols (a) and flavonoids (b) in A. triphylla leaves affected by supplementary light source: HPS, high pressure sodium; MH, metal halide; FR, far-red; LED-w, white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes. Data are presented as the mean ± standard error of mean (n = 3). Different letters (A and B) indicate significant differences (p < 0.05) among treatments.
3.7. Localization of H2O2 by DAB Staining and Activities of Antioxidant Enzymes
In order to evaluate the level of abiotic stress under different supplementary light sources, the H2O2 in leaves were localized. As shown in Figure 6, the leaf in the FR showed a relatively deep brown appearance, followed by the HPS, implying a high level of H2O2 in leaf cells. Meanwhile, an alleviated symptom was found in the MH and LED-w treatments as compared with other treatments. However, the leaf grown in the LED-mix exhibited a light brown feature, suggesting less stress from the light spectrum and a better condition for growth.
Figure 6.
Histochemical localization of hydrogen peroxide by DAB staining in A. triphylla leaves grown under different supplementary light sources: HPS, high pressure sodium; MH, metal halide; FR, far-red; LED-w, white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes.
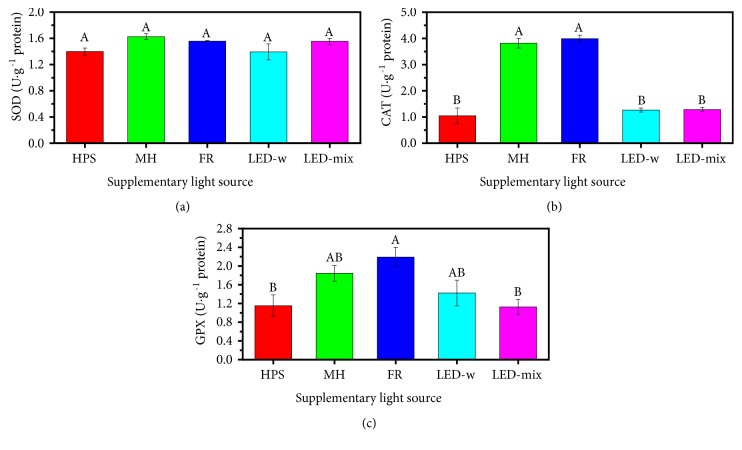
In order to evaluate the scavenging activity of ROS, activities and expressions of antioxidant enzymes such as SOD, CAT, and GPX were measured as shown in Figure 7. The results showed no differences in the SOD activity although the value in the MH and FR treatments was slightly greater than in other treatments (Figure 7(a)). However, the MH and FR significantly promoted the activities of CAT (3.81±0.18 and 3.99±0.14 U·g−1 protein, respectively) as compared to the other three treatments (Figure 7(b)) (p < 0.05). Similarly, the highest activity of GPX was observed in the FR treatment (2.19±0.21 U·g−1 protein), followed by the MH and LED-w, whereas the LED-mix (1.13±0.16 U·g−1 protein) and HPS (1.15±0.23 U·g−1 protein) had remarkably lower values as compared with the FR (Figure 7(c)).
Figure 7.
Activities of SOD (a), CAT (b), and GPX (c) in A. triphylla leaves grown under different supplementary light sources: HPS, high pressure sodium; MH, metal halide; FR, far-red; LED-w, white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes. SOD, sodium dismutase; CAT, catalase; and GPX, guaiacol peroxidase. Data are presented as the mean ± standard error of mean (n = 3). Different letters (A and B) indicate significant differences (p < 0.05) among treatments.
4. Discussion
Supplementary light source is of importance in indoor farming because different light lamps provide specific spectra and greatly influence the quality and quantity of crops grown under those lamps [44, 45]. Thus, choosing the optimal light source is crucial for maximizing crop productivity. [46]. Traditionally, the HPS and MH catch the growers' attention, since these two lamps provide broad ranges of light with lower prices (Figure 1). However, one main disadvantage is the low photoelectric conversion efficiency and higher electricity costs [19, 47]. The green light accounted for more than 40% of the HPS and MH (Table 1), which is far away from the optimum light quality for plant growth and development. Previous studies have established that red and blue light can be perceived by photoreceptors, such as phytochrome, cryptochrome, phototropins, and members of the Zeitlupe family, and be absorbed more efficiently by the plant for photosynthesis [11, 18, 48–51]. Therefore, in order to improve the electron transfer efficiency and to provide special bands for plant growth and development, the LED was introduced and employed in horticulture production [27, 52]. Our data showed that a total of red and blue light in the LED-mix took up 87.5%, which is higher than that in other treatments (Figure 1(f)) and may benefit photosynthesis in leaves and thus for growth and development of seedlings.
The supplementary light source had various effects on growth, development, and morphology of A. triphylla seedlings (Figure 2) [31]. The data showed that the LED-mix improved the quality of seedlings by increasing stem diameter, biomass, specific leaf weight, and root to shoot ratio (Table 2). The probable reason was that LED-mix could provide a higher percentage of red and blue light, increasing light use efficiency for photosynthesis, as compared with other supplementary light sources. Furthermore, high proportions of red and blue light also benefit the development of stomata and photosynthetic components (Figures 3 and 4), which improve photosynthesis and thus seedling quality. The results are in agreement with those in previous studies [10, 33, 53, 54]. However, some studies reported contrary results [24, 29, 55]. Bergstrand et al. [24] reported that tomato and rose crops were improved under the HPS in terms of biomass, plant height, and leaf area. Shao et al. [29] showed a low dry weight in Gynura bicolor under the LEDs as compared with the HPS and T5 fluorescent lamps. Alsanius et al. [55] found that all growth parameters were lower in sunflowers (Helianthus annuus L.) exposed to the LEDs than the HPS. Several factors could have contributed to these differences in results. The first reason probably is the difference in species, since different species have variation in photosensitivity [30, 31]. Secondly, temperatures used in their experiment (about 18 or 20°C in the daytime) are lower than those (31.6/26.3°C day/night) used in our study and the HPS can raise leaf temperature which may be beneficial for plant growth and development in relatively low temperatures [55–57]. As compared with the LED-w, a positive effect on stem diameter and root biomass was found in the LED-mix, because of an increased red and a decreased green light portion (Figure 1(f)). However, these results are consistent with others [58–60]. For example, Bian et al. [58] showed that RB LED (R:B = 4:1) was more effective than the white LED in improving lettuce growth. Zhang et al. [60] found that green light and yellow light inhibited the growth of lettuce. Nawaz et al. [59] found that red light enhanced, while green light suppressed, radicle growth in Brassica rapa.
Stomata are of importance in plant functioning, since they control gas exchange with the atmosphere and influence two basic physiological processes, photosynthesis and transpiration [61–63]. Previous studies found that supplementary light source affected formation, development, and functioning of stomata [17, 32, 64]. Stomatal density and aperture are two limiting factors in plant growth and are also two important indicators of plant adaptation and acclimation to environment [35, 65]. In our study, leaves grown in the LED-mix had greatest stomatal density, implying that the LED-mix improved the formation and development of stomata (Table 4). A high percentage of blue light in the LED-mix might have contributed to a high stomatal density, since blue light was reported to be the most beneficial on the aperture and number of stomata [66]. Zheng et al. [9] presented that, as compared with red light, blue light increased stomatal density and stomatal index and enhanced stomatal conductance in Cordyline australis, Ficus benjamina, and Sinningia speciosa. In addition, a low percentage of green light in the LED-mix might have a positive effect on stomatal density. Jensn et al. [17] reported that stomatal density increased with increasing ratio of blue light, while it decreased with green light in Ocimum basilicum L. Importantly, an increase in stomatal density can improve the gas exchange rate, leaf photosynthetic capacity, and photosynthetic rate [11, 36, 67, 68]. This partly explains enhancement of seedling quality of A. triphylla found in our study. On the other hand, stomatal aperture is a limiting factor in photosynthesis and plant growth [65]. However, our data showed a minimum value in the LED-mix (Table 4). Similarly, Zu et al. [69] found that supplemental UV-B radiation increased stomatal density, while decreasing stomata aperture in Taxus chinensis var. Mairei. Although stomatal aperture in the LED-mix was low in this study, the pore area per leaf area was maintained at a high level which ensured sufficient substrates and suitable gas exchange rate for photosynthesis (Table 4), indicating a fine regulation of gas exchange.
As an important environmental factor, light greatly influences photosynthesis and thus yield since plant growth and yield depend on photosynthesis [6, 7, 12]. Obviously, enhancement of photosynthetic efficiency is of vital importance to increase crop productivity to meet a rising demand of human [70]. The photosynthetic apparatuses, located in the thylakoid, include several integral membrane protein complexes, such as photosystem I (PSI), photosystem II (PSII), cytochrome b6/f, and ATP synthase [13, 71]. The PSII reaction center protein D1, encoded by the psbA gene, plays a key role in the initiation of photosynthesis and photosynthetic electron transport and thus greatly affects photosynthetic efficiency [13, 72, 73]. In our research, the greatest abundance of D1 protein (PsbA) was observed in the LED-mix, implying a higher level of photosynthetic efficiency as compared with others (Figure 4). Moreover, chlorophyll content in the LED-mix was also higher than that in the others, suggesting a stronger capacity for photosynthesis [31]. As a green pigment, chlorophyll is an essential compound of light-harvesting complex (LHC) in the PSI and PSII, which absorb photons and transfer light energy to the reaction center of photosystems [71, 74]. Hence, an increase in chlorophyll content in the LED-mix indicates enhancement of photosynthetic apparatus integrity and light-harvesting efficiency [8]. Efficient absorption and transfer of light energy by the chlorophyll increase the photosynthetic efficiency and then enhance photosynthesis. Thus, the chlorophyll content is closely related to the photosynthetic capacity [75]. Furthermore, as the first key enzyme in carbon fixation, Rubisco was improved in LED-mix, which obviously benefitted the photosynthesis process (Figure 4). Therefore, LED-mix enhanced the production of photosynthetic components such as chlorophyll, D1, and Rubisco, leading to an improved photosynthesis and thus a high quality of seedlings.
Supplementary light source affected accumulation of primary metabolites [20, 28]. Our data showed that the LED-mix enhanced contents of soluble sugar and starch (Table 3), which was in line with the accumulation of biomass (Table 2). The main reason is that the LED-mix could enhance the capacity of photosynthesis by increasing chlorophyll content and expressions of D1 and Rubisco (Figure 4), as compared with other treatments. As a photosynthetic product synthesized by photosynthetic process and a substrate consumed by the respiration, carbohydrate accumulation including soluble sugar and starch is important in plant growth, development, and morphology (Figure 2). Similar results have been found in other studies. For example, as compared with the plants grown in the HPS, plants grown in the LED exhibited 20% higher capacity of photosynthesis and higher levels of soluble carbohydrates in Rosa x hybrida leaves [31]. Moreover, Mao et al. [76] reported that a mixed LED (8R2B) had the highest levels of carbohydrates and lipids and largely promoted the growth of Arthrospira platensis as compared with the white LED. Similarly, combinations of R and B significantly stimulated carbohydrate accumulation in tomato (Solanum lycopersicum) [77], Doritaenopsis [78], Ageratum houstonianum, Tagetes erecta, and Salvia splendens [16], as compared with the white LED, monochromic LED, or fluorescent light.
The accumulation of secondary metabolites is also known to be regulated by supplementary light source [15, 79]. In our study, the highest levels of total phenols and flavonoids were found in the LED-mix (Figure 5). A number of studies showed a similar trend. For instance, Ouzounis [20] found that a 40% B+60% R LED increased contents of all phenolic acids and flavonoids in Rosa hybrida, Chrysanthemum morifolium, and Campanula portenschlagiana as compared with the white or monochromatic LED. It also worked in a similar way in Brassica rapa [59], amaranth (Amaranthus spp.) sprouts [80], and strawberry fruit [81]. The reason is that high blue light in LED-mix greatly contributes to the increase of the secondary metabolites such as phenols and flavonoids as compared with other treatments [26, 82, 83]. More studies suggested that blue light should be recommended as a supplementary light source for accumulation of phenols and flavonoids [84, 85]. Moreover, the promotion of primary metabolism partly promoted contents of phenols and flavonoids in the LED-mix treatment (Table 3), since the products of primary metabolism are utilized to synthesize a wide range of secondary metabolites through the shikimate pathway, mevalonic acid pathway, and methylerythritol 4-phosphate (MEP) pathway in higher plants [86–89]. Results from Zhao et al. [90] showed that enhanced accumulation of most secondary metabolites by elevated temperature is possibly due to increased contents of chlorophyll, sugar, and starch in Robinia pseudoacacia seedlings.
The relationship between reactive oxygen species (ROS) and abiotic stresses in the plant has been well documented [41, 71, 91]. In our research, the localization of H2O2 in leaves showed that oxidation stress increased in the FR and HPS, followed by the MH and LED-w, while it decreased in the LED-mix treatment (Figure 6). A high level of ROS is destructive for photosynthesis, because ROS reduces the abundance of D1 protein in leaves by suppressing biosynthesis and inducing degradation of D1 protein [72, 92]. This partly explains a high level of D1 protein in the LED-mix with a low level of H2O2 (Figures 4 and 6). Moreover, increased ROS also leads to oxidative damage to DNA structure and cell membrane, subsequently leading to a physiological disorder [41, 91, 93], although low levels of ROS play a key role in developmental processes of plants by acting as a signal molecule [93, 94]. In our study, DAB staining showed a low level of H2O2 in the LED-mix treatment, suggesting that the LED-mix provided a more suitable light spectrum (red and blue light) for growth of A. triphylla seedlings. In order to scavenge excessive ROS and to protect them against oxidative damage, plants have evolved efficient enzymatic and nonenzymatic antioxidant systems [40, 57, 91]. In the enzymatic antioxidant system, the SOD catalyzes superoxide radicals into H2O2, which is further degraded into water and oxygen by the CAT or GPX. In this research, it was found that the activities of antioxidant enzymes were associated with oxidative stresses. For example, CAT activity is lower in plants grown under LED-w and LED-mix (Figure 7), while H2O2 accumulation seems to be lower in those treatments (Figure 6). One possible reason might be that plants grown under LED-mix have higher contents of photosynthetic components and thus would be able to use more light energy to drive electron transport to generate ATP and NADPH without dissipation of excess energy [95], leading to lower contents of antioxidant systems. Increases in the activities of antioxidant enzymes under oxidative stresses have been observed in other plants such as Dianthus caryophyllus [34], Trifolium repens [96], and Solanum lycopersicum [97]. In the nonenzymatic antioxidants system, total phenols and flavonoids are crucial compounds for ROS homeostasis in plants, because of a special structure donating electrons or hydrogens [98, 99]. In our study, higher contents of total phenols and flavonoids in the LED-mix might partly contribute to detoxification of ROS and to maintain the level of H2O2 low, thereby protecting seedlings against oxidative damage [20, 29]. Therefore, the LED-mix provided a beneficial light spectrum for growth and development of A. triphylla seedlings with a ROS homeostasis maintained by enzymatic and nonenzymatic antioxidant systems.
5. Conclusions
Supplementary light source is a pivotal factor influencing the quality and yield of crops, thus greatly affecting the interest of growers in commercial cultivations. This study demonstrated the effects of different supplementary light sources on the growth, metabolism, and physiology of A. triphylla seedlings. Our data suggest that the LED-mix, with a higher ratio of red and blue light, has greatly improved the quality of A. triphylla seedlings with a high biomass, compact stem, and well-developed roots. Moreover, the LED-mix significantly promoted the accumulation of primary and secondary metabolites, such as soluble sugar, starch, chlorophyll, total phenols, and flavonoids. Furthermore, results of an SEM analysis implied that the LED-mix has increased stomatal density and maintained a higher level of pore area per leaf area. Meanwhile the Western blot analysis suggested that the expression of photosynthetic protein, D1, was also promoted in the LED-mix. Additionally, the LED-mix mitigated the oxidative damage by maintaining a redox homeostasis regulated by enzymatic and nonenzymatic antioxidant systems. Collectively, the LED-mix was an alternative supplementary light source for the production of high quality A. triphylla seedlings.
Acknowledgments
This study was carried out with the support of the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (Project no. 116057-03). Ya Liu and Xiuxia Ren were supported by a scholarship from the BK21 Plus Program, Ministry of Education, Republic of Korea.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Conceptualization was done by Ya Liu, Xiuxia Ren, and Byoung Ryong Jeong; funding acquisition was done by Byoung Ryong Jeong; investigation was done by Ya Liu and Xiuxia Ren; project administration was done by Xiuxia Ren and Byoung Ryong Jeong; supervision was done by Byoung Ryong Jeong; Ya Liu wrote the original draft; Ya Liu, Xiuxia Ren, and Byoung Ryong Jeong reviewed and edited the manuscript.
Supplementary Materials
Figure S1: Expression of chloroplast proteins (A) and relative contents (B-G) in leaves of A. triphylla seedlings grown under different supplementary light sources analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). HPS, high pressure sodium; MH, metal halide; FR, far-red; LED-w, white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes. The extracted proteins for each sample were loaded on the gel using an equal soluble protein basis. The contents of proteins were shown as a percentage relative to HPS. Band 10, Rubisco large subunit; Band 29, Rubisco small subunit.
References
- 1.Kim J.-H., Hong J.-Y., Shin S.-R., Yoon K.-Y. Comparison of antioxidant activity in wild plant (Adenophora triphylla) leaves and roots as a potential source of functional foods. International Journal of Food Sciences and Nutrition. 2009;60(2):150–161. doi: 10.1080/09637480902956594. [DOI] [PubMed] [Google Scholar]
- 2.Chen C.-C., Chen S.-J., Sagare A. P., Tsay H.-S. Adventitious shoot regeneration from stem internode explants of Adenophora triphylla (Thunb.) A. DC. (Campanulaceae) - An important medicinal herb. Botanical Bulletin of Academia Sinica. 2001;42(1):1–7. [Google Scholar]
- 3.Lee S.-E., Lee E.-H., Lee T.-J., Kim S.-W., Kim B.-H. Anti-obesity effect and action mechanism of adenophora triphylla root ethanol extract in C57BL/6 obese mice fed a high-fat diet. Bioscience, Biotechnology, and Biochemistry. 2013;77(3):544–550. doi: 10.1271/bbb.120667. [DOI] [PubMed] [Google Scholar]
- 4.Chun J., Kang M., Kim Y. S. A triterpenoid saponin from Adenophora triphylla var. japonica suppresses the growth of human gastric cancer cells via regulation of apoptosis and autophagy. Tumor Biology. 2014;35(12):12021–12030. doi: 10.1007/s13277-014-2501-0. [DOI] [PubMed] [Google Scholar]
- 5.Ahn E.-K., Oh J. S. Lupenone isolated from Adenophora triphylla var. japonica extract inhibits adipogenic differentiation through the downregulation of PPARγ in 3T3-L1 Cells. Phytotherapy Research. 2013;27(5):761–766. doi: 10.1002/ptr.4779. [DOI] [PubMed] [Google Scholar]
- 6.Yamori W., Shikanai T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annual Review of Plant Biology. 2016;67:81–106. doi: 10.1146/annurev-arplant-043015-112002. [DOI] [PubMed] [Google Scholar]
- 7.Yamori W., Kondo E., Sugiura D., Terashima I., Suzuki Y., Makino A. Enhanced leaf photosynthesis as a target to increase grain yield: Insights from transgenic rice lines with variable Rieske FeS protein content in the cytochrome b6/f complex. Plant, Cell & Environment. 2016;39(1):80–87. doi: 10.1111/pce.12594. [DOI] [PubMed] [Google Scholar]
- 8.Song Y., Jiang C., Gao L. Polychromatic supplemental lighting from underneath canopy is more effective to enhance tomato plant development by improving leaf photosynthesis and stomatal regulation. Frontiers in Plant Science. 2016;7(2016) doi: 10.3389/fpls.2016.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng L., Van Labeke M.-C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Frontiers in Plant Science. 2017;8 doi: 10.3389/fpls.2017.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macedo A. F., Leal-Costa M. V., Tavares E. S., Lage C. L. S., Esquibel M. A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environmental and Experimental Botany. 2011;70(1):43–50. doi: 10.1016/j.envexpbot.2010.05.012. [DOI] [Google Scholar]
- 11.Wang J., Lu W., Tong Y., Yang Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Frontiers in Plant Science. 2016;7(250) doi: 10.3389/fpls.2016.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Xu H., Shao L., Li T., Wang Y., Wang R. Response of photosynthetic capacity of tomato leaves to different LED light wavelength. Environmental and Experimental Botany. 2018;150:161–171. doi: 10.1016/j.envexpbot.2018.03.013. [DOI] [Google Scholar]
- 13.Jin H., Fu M., Duan Z., et al. Low photosynthetic efficiency 1 is required for light-regulated photosystem II biogenesis in Arabidopsis. Proceedings of the National Acadamy of Sciences of the United States of America. 2018;115(26):E6075–E6084. doi: 10.1073/pnas.1807364115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabara R. C., Behrman G., Timbol T., Rushton P. J. Effect of spectral quality of monochromatic LED lights on the growth of Artichoke seedlings. Frontiers in Plant Science. 2017;8(190) doi: 10.3389/fpls.2017.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szopa A., Starzec A., Ekiert H. The importance of monochromatic lights in the production of phenolic acids and flavonoids in shoot cultures of Aronia melanocarpa, Aronia arbutifolia and Aronia x prunifolia. Journal of Photochemistry and Photobiology B: Biology. 2018;179:91–97. doi: 10.1016/j.jphotobiol.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Jeong W. H., Chun W. L., Kee Y. P. Influence of mixed LED radiation on the growth of annual plants. Journal of Plant Biology. 2006;49(4):286–290. doi: 10.1007/BF03031157. [DOI] [Google Scholar]
- 17.Jensen N. B., Clausen M. R., Kjaer K. H. Spectral quality of supplemental LED grow light permanently alters stomatal functioning and chilling tolerance in basil (Ocimum basilicum L.) Scientia Horticulturae. 2018;227:38–47. doi: 10.1016/j.scienta.2017.09.011. [DOI] [Google Scholar]
- 18.Hogewoning S. W., Trouwborst G., Maljaars H., Poorter H., van Ieperen W., Harbinson J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. Journal of Experimental Botany. 2010;61(11):3107–3117. doi: 10.1093/jxb/erq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L., Van Labeke M.-C. Effects of different irradiation levels of light quality on Chrysanthemum. Scientia Horticulturae. 2018;233:124–131. doi: 10.1016/j.scienta.2018.01.033. [DOI] [Google Scholar]
- 20.Ouzounis T., Fretté X., Rosenqvist E., Ottosen C.-O. Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. Journal of Plant Physiology. 2014;171(16):1491–1499. doi: 10.1016/j.jplph.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Wollaeger H. M., Runkle E. S. Growth and acclimation of impatiens, salvia, petunia, and tomato seedlings to blue and red light. HortScience. 2015;50(4):522–529. doi: 10.21273/HORTSCI.50.4.522. [DOI] [Google Scholar]
- 22.Li Q., Kubota C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environmental and Experimental Botany. 2009;67(1):59–64. doi: 10.1016/j.envexpbot.2009.06.011. [DOI] [Google Scholar]
- 23.Paradiso R., Meinen E., Snel J. F. H., et al. Spectral dependence of photosynthesis and light absorptance in single leaves and canopy in rose. Scientia Horticulturae. 2011;127(4):548–554. doi: 10.1016/j.scienta.2010.11.017. [DOI] [Google Scholar]
- 24.Bergstrand K.-J., Mortensen L. M., Suthaparan A., Gislerød H. R. Acclimatisation of greenhouse crops to differing light quality. Scientia Horticulturae. 2016;204:1–7. doi: 10.1016/j.scienta.2016.03.035. [DOI] [Google Scholar]
- 25.Park Y., Runkle E. S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environmental and Experimental Botany. 2017;136:41–49. doi: 10.1016/j.envexpbot.2016.12.013. [DOI] [Google Scholar]
- 26.Duarte J. H., Costa J. A. V. Blue light emitting diodes (LEDs) as an energy source in Chlorella fusca and Synechococcus nidulans cultures. Bioresource Technology. 2018;247:1242–1245. doi: 10.1016/j.biortech.2017.09.143. [DOI] [PubMed] [Google Scholar]
- 27.Hasan M. M., Bashir T., Ghosh R., Lee S. K., Bae H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules. 2017;22(9) doi: 10.3390/molecules22091420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren X., Liu Y., Jeong H. K., Jeong B. R. Supplementary light source affects the growth and development of Codonopsis lanceolata seedlings. International Journal of Molecular Sciences. 2018;19(10) doi: 10.3390/ijms19103074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao L., Fu Y., Liu H., Liu H. Changes of the antioxidant capacity in Gynura bicolor DC under different light sources. Scientia Horticulturae. 2015;184:40–45. doi: 10.1016/j.scienta.2014.12.010. [DOI] [Google Scholar]
- 30.Bagdonaviciene A., Brazaityte A., Virsile A., et al. Cultivation of sweet pepper (Capsicum annuum L.) transplants under high pressure sodium lamps supplemented by light-emitting diodes of various wavelengths. Acta Scientiarum Polonorum-Hortorum Cultus. 2015;14(6):3–14. [Google Scholar]
- 31.Terfa M. T., Solhaug K. A., Gislerød H. R., Olsen J. E., Torre S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa x hybrida but does not affect time to flower opening. Physiologia Plantarum. 2013;148(1):146–159. doi: 10.1111/j.1399-3054.2012.01698.x. [DOI] [PubMed] [Google Scholar]
- 32.do Nascimento Vieira L., de Freitas Fraga H. P., dos Anjos K. G., et al. Light-emitting diodes (LED) increase the stomata formation and chlorophyll content in Musa acuminata (AAA) ‘Nanicão Corupá’ in vitro plantlets. Theoretical and Experimental Plant Physiology. 2015;27(2):91–98. doi: 10.1007/s40626-015-0035-5. [DOI] [Google Scholar]
- 33.Li H., Xu Z., Tang C. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell, Tissue and Organ Culture. 2010;103(2):155–163. doi: 10.1007/s11240-010-9763-z. [DOI] [Google Scholar]
- 34.Soundararajan P., Manivannan A., Cho Y. S., Jeong B. R. Exogenous supplementation of silicon improved the recovery of hyperhydric shoots in Dianthus caryophyllus L. by stabilizing the physiology and protein expression. Frontiers in Plant Science. 2017;8(738) doi: 10.3389/fpls.2017.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sack L., Buckley T. N. The developmental basis of stomatal density and flux. Plant Physiology. 2016;171(4):2358–2363. doi: 10.1104/pp.16.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monda K., Araki H., Kuhara S., et al. Enhanced stomatal conductance by a spontaneous Arabidopsis tetraploid, Me-0, results from increased stomatal size and greater stomatal aperture. Plant Physiology. 2016;170(3):1435–1444. doi: 10.1104/pp.15.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doheny-Adams T., Hunt L., Franks P. J., Beerling D. J., Gray J. E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367(1588):547–555. doi: 10.1098/rstb.2011.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue J., Wang S., Zhang P., et al. On the role of physiological substances, abscisic acid and its biosynthetic genes in seed maturation and dormancy of tree peony (Paeonia ostii 'Feng Dan') Scientia Horticulturae. 2015;182:92–101. doi: 10.1016/j.scienta.2014.11.021. [DOI] [Google Scholar]
- 39.Liu Y., Ren X., Jeong H. K., Wei H., Jeong B. R. Growth and physiological responses of Adenophora triphylla (Thunb.) A.DC. plug seedlings to day and night temperature regimes. Agronomy. 2018;8(9):p. 173. [Google Scholar]
- 40.Manivannan A., Soundararajan P., Park Y. G., Jeong B. R. In vitro propagation, phytochemical analysis, and evaluation of free radical scavenging property of Scrophularia kakudensis Franch tissue extracts. BioMed Research International. 2015;2015:11. doi: 10.1155/2015/480564.480564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muneer S., Soundararajan P., Jeong B. R. Proteomic and antioxidant analysis elucidates the underlying mechanism of tolerance to hyperhydricity stress in in vitro shoot cultures of Dianthus caryophyllus. Journal of Plant Growth Regulation. 2016;35(3):667–679. doi: 10.1007/s00344-015-9569-7. [DOI] [Google Scholar]
- 42.Muneer S., Park Y. G., Jeong B. R. Red and blue light emitting diodes (LEDs) participate in mitigation of hyperhydricity in in vitro-grown carnation genotypes (Dianthus caryophyllus) Journal of Plant Growth Regulation. 2018;37(2):370–379. doi: 10.1007/s00344-017-9733-3. [DOI] [Google Scholar]
- 43.Muneer S., Kim E. J., Park J. S., Lee J. H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.) International Journal of Molecular Sciences. 2014;15(3):4657–4670. doi: 10.3390/ijms15034657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogewoning S. W., Douwstra P., Trouwborst G., Van Ieperen W., Harbinson J. An artificial solar spectrum substantially alters plant development compared with usual climate room irradiance spectra. Journal of Experimental Botany. 2010;61(5):1267–1276. doi: 10.1093/jxb/erq005. [DOI] [PubMed] [Google Scholar]
- 45.Amoozgar A., Mohammadi A., Sabzalian M. R. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica. 2017;55(1):85–95. doi: 10.1007/s11099-016-0216-8. [DOI] [Google Scholar]
- 46.Ye S., Shao Q., Xu M., et al. Effects of light quality on morphology, enzyme activities, and bioactive compound contents in Anoectochilus roxburghii. Frontiers in Plant Science. 2017;8 doi: 10.3389/fpls.2017.00857.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dutta Gupta S., Jatothu B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnology Reports. 2013;7(3):211–220. doi: 10.1007/s11816-013-0277-0. [DOI] [Google Scholar]
- 48.He J., Qin L., Chong E. L. C., Choong T.-W., Lee S. K. Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue- and red-LEDs. Frontiers in Plant Science. 2017;8(361) doi: 10.3389/fpls.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goins G. D., Yorio N. C., Sanwo M. M., Brown C. S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. Journal of Experimental Botany. 1997;48(312):1407–1413. doi: 10.1093/jxb/48.7.1407. [DOI] [PubMed] [Google Scholar]
- 50.Christie J. M. Phototropin blue-light receptors. Annual Review of Plant Biology. 2007;58(1):21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- 51.Suetsugu N., Wada M. Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: Phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant & Cell Physiology (PCP) 2013;54(1):8–23. doi: 10.1093/pcp/pcs165. [DOI] [PubMed] [Google Scholar]
- 52.Bantis F., Smirnakou S., Ouzounis T., Koukounaras A., Ntagkas N., Radoglou K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs) Scientia Horticulturae. 2018;235:437–451. doi: 10.1016/j.scienta.2018.02.058. [DOI] [Google Scholar]
- 53.Hung C. D., Hong C.-H., Jung H.-B., et al. Growth and morphogenesis of encapsulated strawberry shoot tips under mixed LEDs. Scientia Horticulturae. 2015;194:194–200. doi: 10.1016/j.scienta.2015.08.016. [DOI] [Google Scholar]
- 54.Talukder M. R., Asaduzzaman M., Tanaka H., Asao T. Light-emitting diodes and exogenous amino acids application improve growth and yield of strawberry plants cultivated in recycled hydroponics. Scientia Horticulturae. 2018;239:93–103. doi: 10.1016/j.scienta.2018.05.033. [DOI] [Google Scholar]
- 55.Alsanius B. W., Bergstrand K.-J., Hartmann R., et al. Ornamental flowers in new light: Artificial lighting shapes the microbial phyllosphere community structure of greenhouse grown sunflowers (Helianthus annuus L.) Scientia Horticulturae. 2017;216:234–247. doi: 10.1016/j.scienta.2017.01.022. [DOI] [Google Scholar]
- 56.Nelson J. A., Bugbee B. Analysis of environmental effects on leaf temperature under sunlight, high pressure sodium and light emitting diodes. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0138930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ribeiro P. R., Zanotti R. F., Deflers C., et al. Effect of temperature on biomass allocation in seedlings of two contrasting genotypes of the oilseed crop Ricinus communis. Journal of Plant Physiology. 2015;185:31–39. doi: 10.1016/j.jplph.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Bian Z.-H., Cheng R.-F., Yang Q.-C., Wang J., Lu C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. Journal of the American Society for Horticultural Science. 2016;141(2):186–195. doi: 10.21273/JASHS.141.2.186. [DOI] [Google Scholar]
- 59.Nawaz T., Ahmad N., Ali S., Khan M., Fazal H., Khalil S. A. Developmental variation during seed germination and biochemical responses of Brassica rapa exposed to various colored lights. Journal of Photochemistry and Photobiology B: Biology. 2018;179:113–118. doi: 10.1016/j.jphotobiol.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Zhang T., Shi Y., Piao F., Sun Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell, Tissue and Organ Culture. 2018;134(2):231–240. doi: 10.1007/s11240-018-1415-8. [DOI] [Google Scholar]
- 61.Hedrich R., Geiger D. Biology of SLAC1-type anion channels - from nutrient uptake to stomatal closure. New Phytologist. 2017;216(1):46–61. doi: 10.1111/nph.14685. [DOI] [PubMed] [Google Scholar]
- 62.Rui Y., Anderson C. T. Functional analysis of cellulose and xyloglucan in the walls of stomatal guard cells of Arabidopsis. Plant Physiology. 2016;170(3):1398–1419. doi: 10.1104/pp.15.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fanourakis D., Giday H., Milla R., et al. Pore size regulates operating stomatal conductance, while stomatal densities drive the partitioning of conductance between leaf sides. Annals of Botany. 2015;115(4):555–565. doi: 10.1093/aob/mcu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee S.-H., Tewari R. K., Hahn E.-J., Paek K.-Y. Photon flux density and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania Somnifera (L.) Dunal. plantlets. Plant Cell, Tissue and Organ Culture. 2007;90(2):141–151. doi: 10.1007/s11240-006-9191-2. [DOI] [Google Scholar]
- 65.Wang Y., Noguchi K., Ono N., Inoue S.-I., Terashima I., Kinoshita T. Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proceedings of the National Acadamy of Sciences of the United States of America. 2014;111(1):533–538. doi: 10.1073/pnas.1305438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simlat M., Ślęzak P., Moś M., Warchoł M., Skrzypek E., Ptak A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Scientia Horticulturae. 2016;211:295–304. doi: 10.1016/j.scienta.2016.09.009. [DOI] [Google Scholar]
- 67.Tanaka Y., Sugano S. S., Shimada T., Hara-Nishimura I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytologist. 2013;198(3):757–764. doi: 10.1111/nph.12186. [DOI] [PubMed] [Google Scholar]
- 68.Roni M. Z., Islam M. S., Shimasaki K. Response of eustoma leaf phenotype and photosynthetic performance to LED light quality. Horticulturae. 2017;3(4):p. 50. doi: 10.3390/horticulturae3040050. [DOI] [Google Scholar]
- 69.Zu Y.-G., Pang H.-H., Yu J.-H., et al. Responses in the morphology, physiology and biochemistry of Taxus chinensis var. mairei grown under supplementary UV-B radiation. Journal of Photochemistry and Photobiology B: Biology. 2010;98(2):152–158. doi: 10.1016/j.jphotobiol.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X.-G., Long S. P., Ort D. R. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology. 2010;61:235–261. doi: 10.1146/annurev-arplant-042809-112206. [DOI] [PubMed] [Google Scholar]
- 71.Gu J., Zhou Z., Li Z., et al. Photosynthetic properties and potentials for improvement of photosynthesis in pale green leaf rice under high light conditions. Frontiers in Plant Science. 2017;8 doi: 10.3389/fpls.2017.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lupínkov L., Komenda J. Oxidative modifications of the photosystem II D1 protein by reactive oxygen species: from isolated protein to cyanobacterial cells. Photochemistry and Photobiology. 2004;79(2):152–162. doi: 10.1562/0031-8655(2004)079<0152:OMOTPI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 73.Barber J., Kühlbrandt W. Photosystem II. Current Opinion in Structural Biology. 1999;9(4):469–475. doi: 10.1016/S0959-440X(99)80066-9. [DOI] [PubMed] [Google Scholar]
- 74.Melis A. Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Journal of Plant Sciences. 2009;177(4):272–280. doi: 10.1016/j.plantsci.2009.06.005. [DOI] [Google Scholar]
- 75.Tewolde F. T., Lu N., Shiina K., et al. Nighttime supplemental LED inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Frontiers in Plant Science. 2016;7(488) doi: 10.3389/fpls.2016.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mao R., Guo S. Performance of the mixed LED light quality on the growth and energy efficiency of Arthrospira platensis. Applied Microbiology and Biotechnology. 2018;102(12):5245–5254. doi: 10.1007/s00253-018-8923-7. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Xin G., Wei M., Shi Q., Yang F., Wang X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Scientia Horticulturae. 2017;225:490–497. doi: 10.1016/j.scienta.2017.07.053. [DOI] [Google Scholar]
- 78.Shin K. S., Murthy H. N., Heo J. W., Hahn E. J., Paek K. Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiologiae Plantarum. 2008;30(3):339–343. doi: 10.1007/s11738-007-0128-0. [DOI] [Google Scholar]
- 79.Dutta Gupta S., Karmakar A. Machine vision based evaluation of impact of light emitting diodes (LEDs) on shoot regeneration and the effect of spectral quality on phenolic content and antioxidant capacity in Swertia chirata. Journal of Photochemistry and Photobiology B: Biology. 2017;174:162–172. doi: 10.1016/j.jphotobiol.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 80.Cho J. Y., Son D. M., Kim J. M., et al. Effects of LEDs on the germination, growth and physiological activities of amaranth sprouts. Korean Journal of Horticultural Science & Technology. 2008;26(2):106–112. [Google Scholar]
- 81.Choi H. G., Kwon J. K., Moon B. Y., et al. Effect of different light emitting diode (LED) lights on the growth characteristics and the phytochemical production of strawberry fruits during Cultivation. Korean Journal of Horticultural Science Technology. 2013;31(1):56–64. [Google Scholar]
- 82.Jeon Y.-M., Son K.-H., Kim S.-M., Oh M.-M. Growth and bioactive compounds as affected by irradiation with various spectrum of light-emitting diode lights in dropwort. Horticulture, Environment, and Biotechnology. 2017;58(5):467–478. doi: 10.1007/s13580-017-0354-3. [DOI] [Google Scholar]
- 83.Nam T. G., Kim D.-O., Eom S. H. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Science and Biotechnology. 2018;27(1):169–176. doi: 10.1007/s10068-017-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu H., Chen Y., Hu T., et al. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. Journal of Functional Foods. 2016;25:459–465. doi: 10.1016/j.jff.2016.06.028. [DOI] [Google Scholar]
- 85.Cuong D. M., Kwon S.-J., Jeon J., Park Y. J., Park J. S., Park S. U. Identification and characterization of phenylpropanoid biosynthetic genes and their accumulation in bitter melon (Momordica charantia) Molecules. 2018;23(2) doi: 10.3390/molecules23020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie L., Liu P., Zhu Z., et al. Phylogeny and expression analyses reveal important roles for plant PKS III family during the conquest of land by plants and angiosperm diversification. Frontiers in Plant Science. 2016;7(2016) doi: 10.3389/fpls.2016.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oliva M., Ovadia R., Perl A., et al. Enhanced formation of aromatic amino acids increases fragrance without affecting flower longevity or pigmentation in Petunia x hybrida. Plant Biotechnology Journal. 2015;13(1):125–136. doi: 10.1111/pbi.12253. [DOI] [PubMed] [Google Scholar]
- 88.Banerjee A., Sharkey T. D. Methylerythritol 4-phosphate (MEP) pathway metabolic regulation. Natural Product Reports. 2014;31(8):1043–1055. doi: 10.1039/c3np70124g. [DOI] [PubMed] [Google Scholar]
- 89.Tzin V., Galili G. New Insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Molecular Plant. 2010;3(6):956–972. doi: 10.1093/mp/ssq048. [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y. H., Jia X., Wang W. K., Liu T., Huang S. P., Yang M. Y. Growth under elevated air temperature alters secondary metabolites in Robinia pseudoacacia L. seedlings in Cd- and Pb-contaminated soils. Science of the Total Environment. 2016;565:586–594. doi: 10.1016/j.scitotenv.2016.05.058. [DOI] [PubMed] [Google Scholar]
- 91.Martinez V., Nieves-Cordones M., Lopez-Delacalle M., et al. Tolerance to stress combination in tomato plants: new insights in the protective role of melatonin. Molecules. 2018;23(3):p. 535. doi: 10.3390/molecules23030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishiyama Y., Allakhverdiev S. I., Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2006;1757(7):742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 93.Singh R., Singh S., Parihar P., et al. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Frontiers in Plant Science. 2016;7(2016) doi: 10.3389/fpls.2016.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeng J., Dong Z., Wu H., Tian Z., Zhao Z. Redox regulation of plant stem cell fate. EMBO Journal. 2017;36(19):2844–2855. doi: 10.15252/embj.201695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamori W. Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. Journal of Plant Research. 2016;129(3):379–395. doi: 10.1007/s10265-016-0816-1. [DOI] [PubMed] [Google Scholar]
- 96.Wang C.-Q., Zhang Y.-F., Zhang Y.-B. Scavenger enzyme activities in subcellular fractions of white clover (Trifolium repens L.) under PEG-induced water stress. Journal of Plant Growth Regulation. 2008;27(4):387–393. doi: 10.1007/s00344-008-9065-4. [DOI] [Google Scholar]
- 97.Ding F., Liu B., Zhang S. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Scientia Horticulturae. 2017;219:264–271. doi: 10.1016/j.scienta.2017.03.029. [DOI] [Google Scholar]
- 98.Reddy N. S., Navanesan S., Sinniah S. K., Wahab N. A., Sim K. S. Phenolic content, antioxidant effect and cytotoxic activity of Leea indica leaves. BMC Complementary and Alternative Medicine. 2012;12(1):p. 128. doi: 10.1186/1472-6882-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng L., Van Labeke M.-C. Chrysanthemum morphology, photosynthetic efficiency and antioxidant capacity are differentially modified by light quality. Journal of Plant Physiology. 2017;213:66–74. doi: 10.1016/j.jplph.2017.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Expression of chloroplast proteins (A) and relative contents (B-G) in leaves of A. triphylla seedlings grown under different supplementary light sources analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). HPS, high pressure sodium; MH, metal halide; FR, far-red; LED-w, white (red: green: blue = 2:4:3) light-emitting diodes; and LED-mix, mixed (red: green: blue = 4:1:4) light-emitting diodes. The extracted proteins for each sample were loaded on the gel using an equal soluble protein basis. The contents of proteins were shown as a percentage relative to HPS. Band 10, Rubisco large subunit; Band 29, Rubisco small subunit.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.