Table 3:
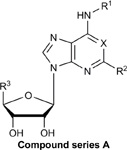
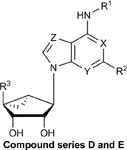
Structures, efficacies (Emax), potencies (logEC50), and hA3AR binding affinities (Ki) of ten additional structural analogs tested in the stable hA3AR-NanoBit®-βarr2 HEK293T cell line.
 |
 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | X | Y | Z | Stable
A3AR NanoBit® HEK293T cell lineA |
Ki (nM) ± SEM | |
| Emax (%) | LogEC50 ± SEM | ||||||||
| A. Ribose 5’-OH analogues | |||||||||
| DCCA | cyclopentyl | Cl | CH2OH | CH | - | - | 5.8 ± 0.2 | −5.83 ± 0.07 | 244± 37 |
| N6-benzyl-adenosine | benzyl | H | CH2OH | N | - | - | 34.9 ± 2.3 | −6.07 ± 0.13 | 41.7 ± 5.3 |
| N6-phenyl-adenosine | phenyl | H | CH2OH | N | - | - | 75.8 ± 3.0 | −6.23 ± 0.0 | 14.9 ± 3.1 |
| D. Methanocarba, 5’-amides, C2-extended | |||||||||
| MRS5679 | 3-Cl-benzyl | (4-phenyl-phenyl)ethynyl | CONHCH3 | N | N | N | 46.8 ± 3.3 | −5.92 ± 0.11 | 3.06 ± 1.35 |
| MRS5967 | CH3 | (2-CH3O-phenyl)ethynyl | CONHCH3 | N | N | N | 92.5 ± 2.4 | −8.33 ± 0.06 | 0.77 ± 0.17 |
| MRS5663 | CH3 | (2-Cl-phenyl)ethynyl | CONHCH3 | N | N | N | 101.7 ± 3.6 | −8.25 ± 0.08 | 0.58 ± 0.04 |
| MRS5980 | CH3 | (5-Cl-thien-2-yl)ethynyl | CONHCH3 | N | N | N | 95.0 ± 4.0 | −8.71 ± 0.11 | 0.7 ± 0.11 |
| MRS7154 | (CH2)2CH3 | (5-Cl-thien-2-yl)ethynyl | CONHCH3 | N | N | N | 94.5 ± 4.8 | −7.67 ± 0.11 | 1.1 ± 0.3 |
| E. Methanocarba, deaza-adenine | |||||||||
| MRS7173 | CH3 | (5-Cl-thien-2-yl)ethynyl | CONHCH3 | N | CH | N | 78.2 ± 1.8 | −7.26 ± 0.04 | 1.56 ± 0.2 |
| MRS7232 | CH3 | (5-Cl-thien-2-yl)ethynyl | CO2C2H5 | N | N | N | 13.6 ± 1.6 | −7.30 ± 0.17 | 5.38 ± 0.03 |
Emax is relative to that of reference agonist NECA
