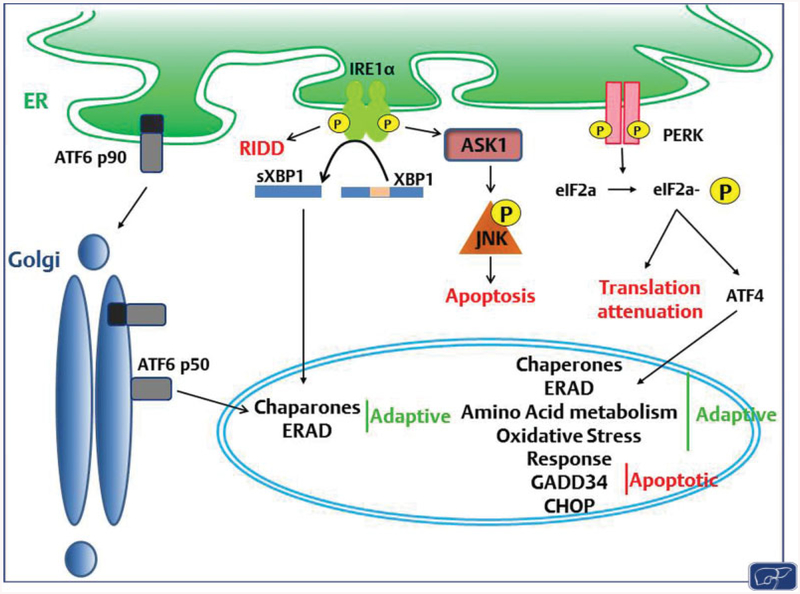
Fig. 1. UPR sensors and signaling pathways.
ER stress is sensed by three ER transmembrane proteins, ATF6α, IRE1α, and PERK, which are crucial for mediating the adaptive and apoptotic signaling of the UPR. ATF6α translocates to the Golgi upon sensing ER stress, where it is cleaved and subsequently trafficked to the nucleus where it upregulates chaperones and proteins involved in ERAD. IRE1α oligomerizes and autophosphorylates in response to ER stress, and acts through several mechanisms. The endonuclease domain is involved in RIDD, as well as transcription through activating the transcription factor XBP1. IRE1α also promotes apoptosis through activation of ASK and subsequent phosphorylation of JNK. PERK oligomerizes upon sensing ER stress, and autophosphorylates. The canonical target of PERK kinase activity is eIF2α, which acts through ATF4 to promote expression of chaperones, and proteins involved in ERAD, amino acid metabolism, the oxidative stress response, and UPR-mediated apoptosis. eIF2α also serves to attenuate nonessential mRNA translation. CHOP, a stress-induced transcription factor, is upregulated downstream of ATF4 and mediates ER stress-induced apoptosis. Gad34, another transcriptional target of this pathway, dephosphorylates eIF2α, thus resuming translation which can lead to apoptosis by increasing oxidative protein folding. ASK, apoptosis-signal-regulating kinase; CHOP, CCAAT-enhancer-binding protein homologous protein; ER, endoplasmic reticulum; ERAD, endoplasmic reticulum-associated degradation; JNK, c-Jun N-terminal kinase; PERK, protein kinase RNA-like endoplasmic reticulum kinase; RIDD, regulated IRE1-dependent decay; UPR, unfolded protein response.