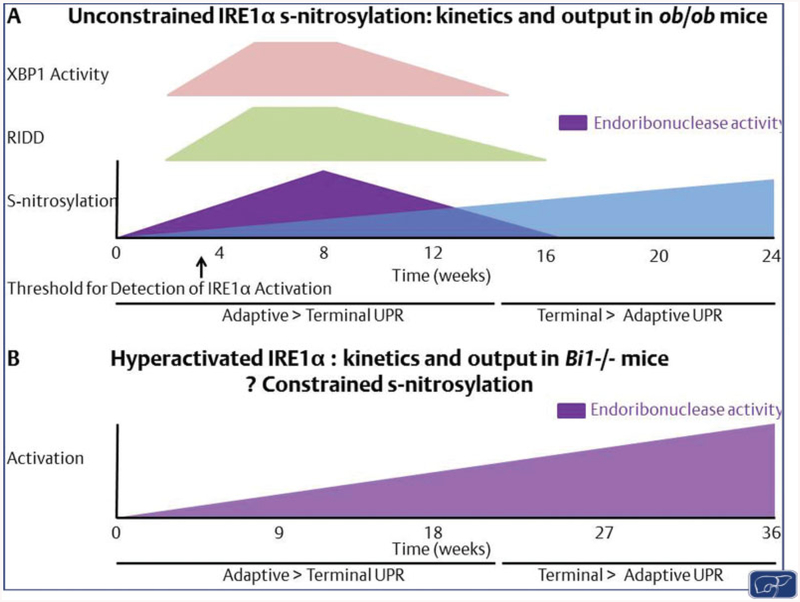
Fig. 2. IRE1α activation states in NAFLD.
In this model we propose two alternative activation states of IRE1α: (A) unconstrained IRE1α snitrosylation as observed in ob/ob mice, which may increase over time. Initial activation of XBP1 (in pink) and RIDD (in green) may favor adaptive UPR. Over time a loss of endoribonuclease activity (in purple) would occur with increased s-nitrosylation (in blue) leading to a loss of adaptive signaling. (B) In the absence of s-nitrosylation IRE1α activation increases over time and shifts from adaptive to maladaptive. However, whether this switch is a function of time; regulated by the activation of alternative signaling pathways such as PERK; a cell- and tissue-specific activation of IRE1α; or specific to accumulated toxic lipids, remain to be determined. NAFLD, nonalcoholic fatty liver disease; PERK, protein kinase RNA-like endoplasmic reticulum kinase; RIDD, regulated IRE1-dependent decay; UPR, unfolded protein response.