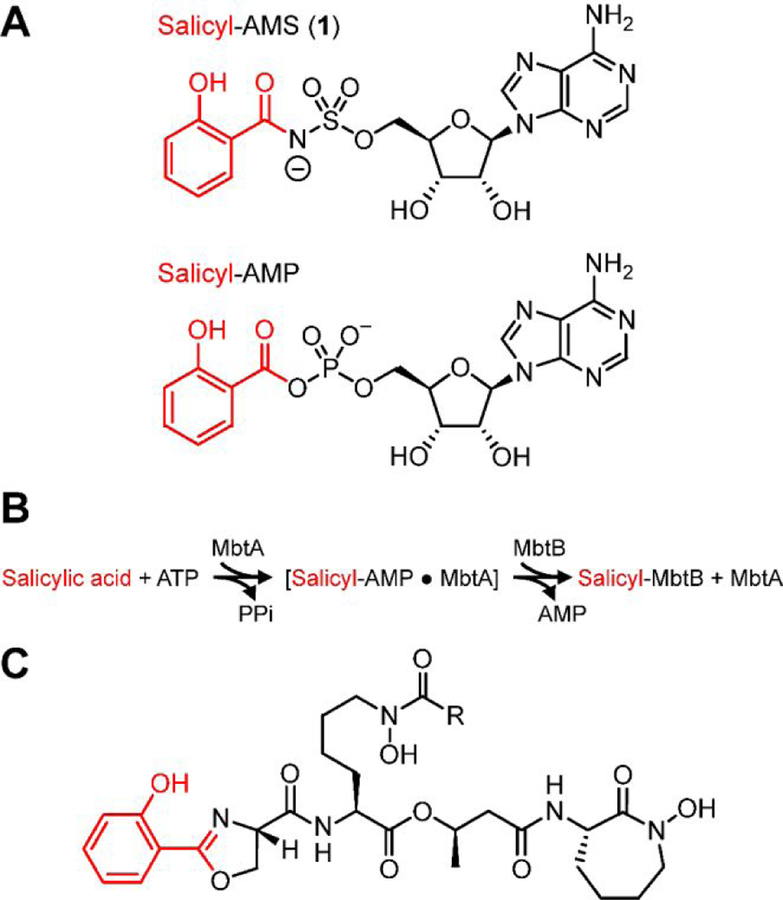
Figure 1.
(A) Nucleoside antibiotic salicyl-AMS and salicyl-AMP intermediate synthesized by the salicylate adenylation enzyme activity of MbtAtb. (B) Reactions catalyzed by MbtAtb during mycobactin (MBT) biosynthesis. MbtAtb catalyzes formation of the first covalent acyl-enzyme intermediate during MBT acyl-chain assembly through a mechanism involving two-half reactions. The first half reaction is the ATP-dependent adenylation of salicylic acid to generate a salicyl-AMP intermediate that remains non-covalently bound to the active site. The second half-reaction is the transfer of the salicyl moiety of the adenylate onto the phosphopantetheinyl group of the carrier protein domain of the peptide synthetase MbtB. (C) Representative mycobactin siderophores of M. tuberculosis. R represents variable fatty acyl groups (mycobactin variants) or acyl substituents terminating in a carboxylate or a methyl ester (carboxymycobactin variants). All of these variants are herein collectively referred to as MBTs.