Figure 3.
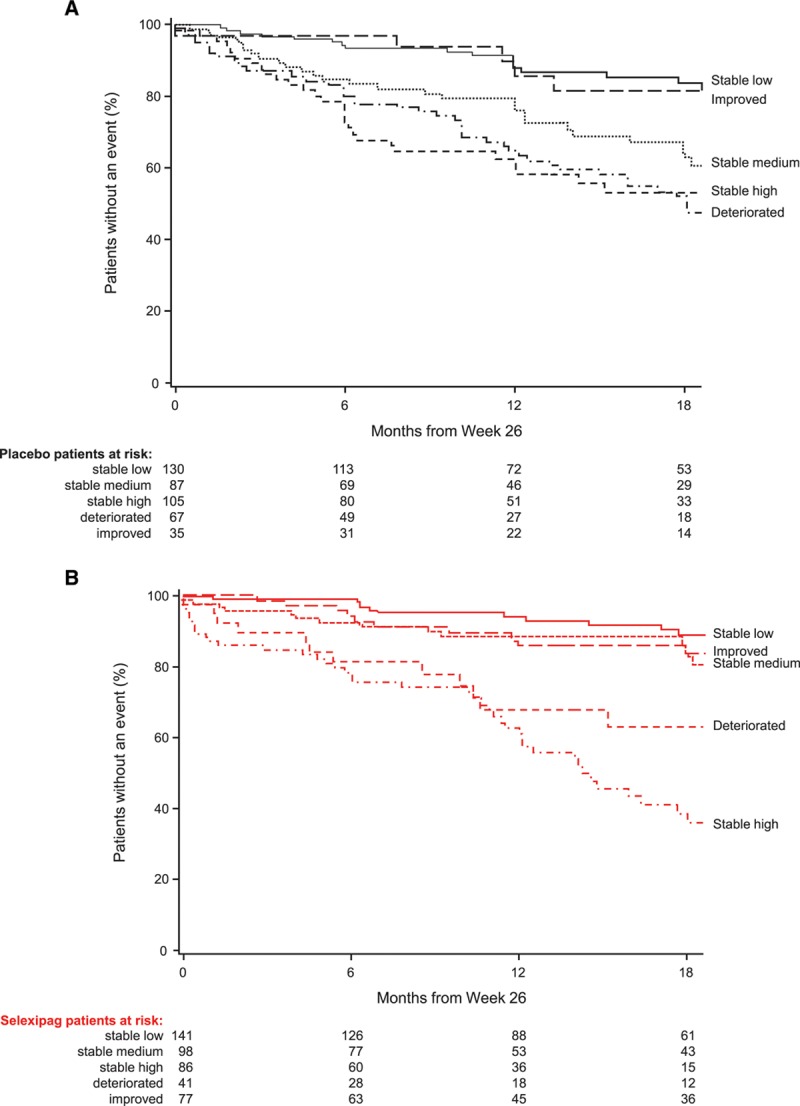
Time from week 26 to first morbidity or mortality event based on change in NT-proBNP (N-terminal pro brain natriuretic peptide) category from baseline to week 26 for placebo and selexipag patients. Data shown for (A) patients receiving placebo and (B) patients receiving selexipag. Low, medium, and high NT-proBNP cutoffs determined according to baseline NT-proBNP tertiles (low: <271 ng/L; medium: 271–1165 ng/L; high: >1165 ng/L). Subgroups are defined as: stable low (low NT-proBNP at baseline and at week 26), stable medium (medium NT-proBNP at baseline and at week 26), stable high (high NT-proBNP at baseline and at week 26), improved (high at baseline and medium or low at week 26, or medium at baseline and low at week 26), and deteriorated (low at baseline and medium or high at week 26, or medium at baseline and high at week 26).
