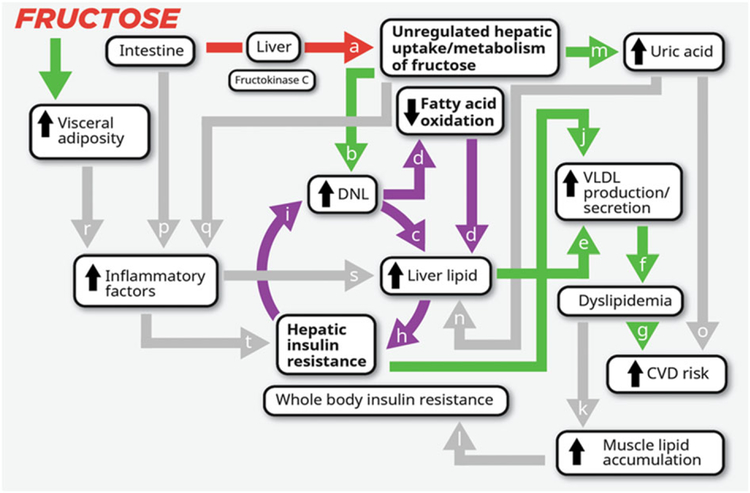
Figure 3.
Potential mechanisms by which consumption of fructose promotes the development of metabolic syndrome The initial phosphorylation of dietary fructose in the liver is largely catalysed by fructokinase C (a), which is not regulated by hepatic energy status (122,123). This results in unregulated fructose uptake and metabolism by the liver. The excess substrate leads to increased de novo lipogenesis (DNL) (b) (117,127). DNL increases the intra-hepatic lipid supply directly (127–129), via synthesis of fatty acids (c), and indirectly by inhibiting fatty acid oxidation (d) (120,127). Increased intra-hepatic lipid content promotes very-low-density lipoprotein (VLDL) production and secretion (e) (130). This leads to increased levels of circulating triglyceride (TG) and low-density lipoprotein particles (dyslipidaemia) (f) (131), risk factors for cardiovascular disease (CVD) (g). Increased levels of hepatic lipid may also promote hepatic insulin resistance (132) by increasing levels of diacylglycerol, which may activate novel protein kinase C and lead to serine phosphorylation (serine P) of the insulin receptor and insulin receptor substrate 1 and impaired insulin action (h) (335). Because of selective insulin resistance, DNL is even more strongly activated in the insulin resistant liver (i) (336), which has the potential to generate a vicious cycle (circular arrows) that would be perpetuated by sustained fructose consumption. This cycle would be expected to further exacerbate VLDL production and secretion via increased intrahepatic lipid supply (130). Hepatic insulin resistance also promotes VLDL production/secretion (j) by increasing apolipoprotein B availability (337,338) and apolipoprotein CIII synthesis (339) and by up-regulating microsomal TG transfer protein expression (MTP) (336). This exacerbates and sustains exposure to circulating TG, leading to intramyocellular lipid accumulation (k) (334), impaired insulin signalling and whole-body insulin resistance (l) (332). The fructokinase-catalysed phosphorylation of fructose to fructose-1-phosphate, which results in conversion of adenosine triphosphate to adenosine monophosphate and a depletion of inorganic phosphate, leads to uric acid production via the purine degradation pathway (m) (121,123–125). High levels of uric acid are associated and may contribute to increased risk for development of fatty liver (n) and CVD (o) (340–342). Fructose exposure in the intestine (p) (134,135) and liver (q) (136) and fructose-induced increases of visceral adipose (r) may promote inflammatory responses (117,343) that further promote liver lipid accumulation (s) and/or impair hepatic insulin signalling (t) (119).