Fig. 2. ATC interactions with TmPPase domains.
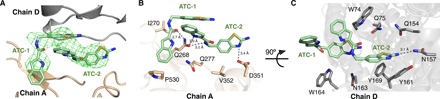
(A) ATC [positive (Fo-Fc) electron density map at 3σ] located on the interface of chains A (wheat) and D (gray) in a hydrophobic cleft. Pale green, carbon; blue, nitrogen; red, oxygen; yellow, sulfur. (B) Residues in chain A that are important for the interactions with the ATC dimer. The closest approach of the two ATC molecules is 3.3 Å, consistent with π-π stacking. (C) Residues on chain D that are important for the interactions with the ATC dimer. Dashed lines represent hydrogen bonds.
