Fig. 2 |. Applications of the protocol.
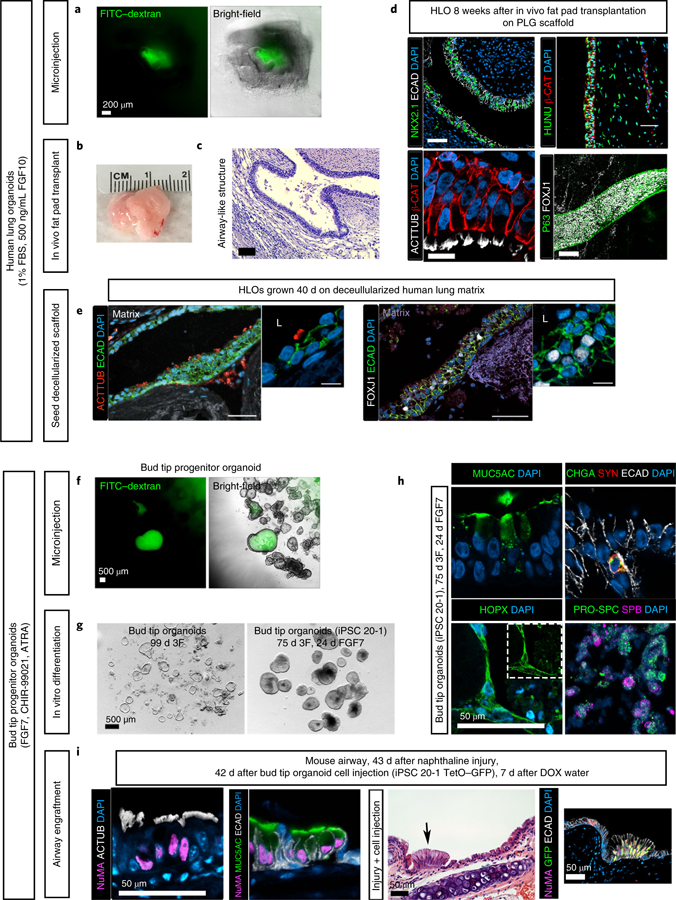
a–e, Potential applications for human lung organoids. a, Organoid lumens can be microinjected, as done here with 1 µL of 4-kDa FITC–dextran suspended in PBS at a concentration of 2 mg/mL (green)20. Images show organoids 1 h after injection. Scale bar, 200 µm (applies to both images). b, Human lung organoids can be seeded on a bioengineered poly(lactic-co-glycolic acid) (PLG) scaffold and transplanted into the mouse fat pad (full methods can be found in ref. 16). This structure can grow to more than 1 cm in diameter in ~8 weeks. c, Transplanted human lung organoids contain airway-like structures, as visualized here by H&E staining after 8 weeks16. Scale bar, 200 µm. d, After 8 weeks of transplantation, human lung organoids (HLOs) contain NKX2.1+ airway epithelial structures that stain positive for human nuclear antigen (HUNU) and exhibit multiciliated cells that stain positive for acetylated tubulin (ACTTUB; a video showing the resulting functional beating ciliated cells is available from ref. 16). Further, these structures contain epithelial tubes with P63+ basal-like cells lining the basolateral surface of the tube and FOXJ1+ ciliated cells lining the interior luminal side of the pseudostratified epithelium. Scale bars, 50 µm. e, Roughly 50 human lung organoids were seeded onto a decellularized human lung matrix punch in a 96-well plate. Human lung organoid seeded cells gave rise to epithelial structures expressing multiciliated-cell markers acetylated tubulin and FOXJ1 after 40 d of culture. Scale bars, 100 µm. f–i, Applications for bud tip progenitor organoids. f, Bud tip progenitor organoids can be microinjected with fluids, as done here with 1 µL of 4-kDa FITC–dextran suspended in PBS at a concentration of 2 mg/ml (green)20. Five organoids were injected. Images show organoids 1 h after injection. Scale bar, 500 µm. g, Bud tip progenitor cells in bud tip progenitor organoids are capable of undergoing multilineage differentiation into airway and alveolar-like cells, and this system can be used to interrogate mechanisms of lineage decisions in vitro. Bud tip progenitor organoids treated for 24 d with FGF7 alone generated cells that expressed the AECI marker HOPX and AECII markers pro-surfactant protein C (PRO-SPC) and surfactant protein B (SFTPB). Alevolar-like cells made up roughly 50% of these organoids. Airway-like cells were also generated, including cells that stained positive for goblet cell marker MUC5AC and neuroendocrine markers chromagranin A (CHGA) and synaptophysin (SYN). Scale bar, 50 µm. i, Bud tip progenitor organoids can be expanded in culture and injected directly into the injured mouse airway, where they can engraft. In this experiment, bud tip progenitor organoids were generated from the iPSC 20–1 tet-O–GFP cell line to provide doxycycline (DOX)-inducible GFP expression. Organoids were grown, expanded and dissociated to single cells, and roughly 500,000 bud tip progenitor organoid cells were injected into the mouse airway 24 h after airway injury with naphthaline. Mice were allowed to recover for 6 weeks. During the fifth week after cell injection, mice were given DOX-treated water; surviving human cells with access to the host blood stream could then begin expressing GFP upon exposure to DOX17. Engrafted cells expressed the human nuclear marker NuMA, as well as markers of differentiation including multiciliated-cell markers (acetylated tubulin) and the goblet cell marker MUC5AC. These cells integrated seamlessly with the host epithelium. Further, cells were able to receive signals from the host bloodstream, as bud tip progenitor cells derived from a tet-O–GFP iPSC line were able to turn on GFP expression when the host mouse was given DOX in drinking water. Scale bars, 50 µm. Detailed information about antibodies used for staining can be found in Table 3. b–d, Reproduced with permission from ref. 16, eLife Sciences Publications (licensed under CC BY-NC-ND 4.0, https://creativecommons.org/licenses/by/4.0/). e, Reproduced with permission from ref. 15, eLife Sciences Publications (licensed under CC BY-NC-ND 4.0, https://creativecommons.org/licenses/by/4.0/). g–i, Reproduced with permission from ref. 17, Elsevier (licensed under CC BY-NC-ND 4.0, https://creativecommons.org/licenses/by/4.0/).
