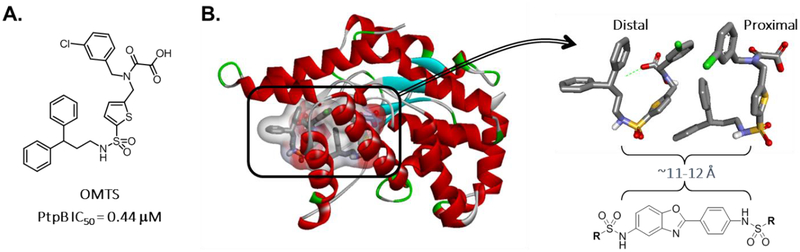
Figure 2.
A. Grundner et al. previously reported that the OMTS compound was a potent and selective inhibitor of M. tuberculosis PtpB.50 B. Two OMTS molecules were found occupying the active site of M. tuberculosis PtpB (PDB ID 2OZ5). On examination, the sulfonamides of each OMTS molecule reside ~11–12 Å apart, similar to our compound 1 analogs. Thus, we envisioned compound 1 analogs might be able to bind in a manner that bridges the distal and proximal parts of the active site. In such a conformation, the sulfonamides could interact with the binding site similarly to those of OMTS, with the R-substructures pointing upwards to fill the binding cavity and engaging the catalytic residues located in the proximal part of the active site.