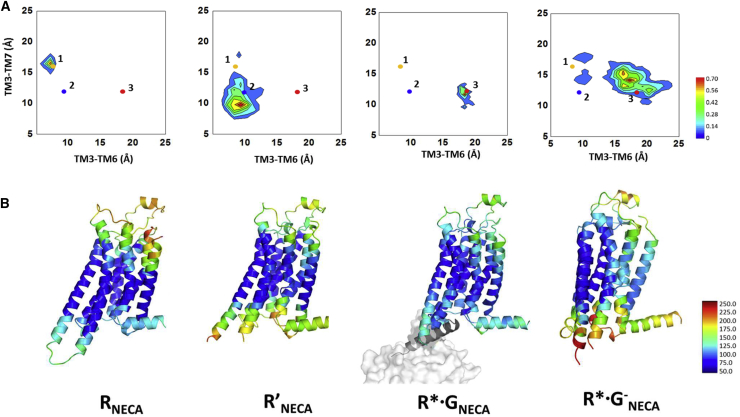
Figure 1.
Conformational Sampling of A2AR Bound to NECA in Four Different States
(A) Conformational ensembles from the MD simulations clustered by comparisons of the distances between TM3-TM6 and TM3-TM7. MD ensembles for A2AR bound to NECA in four different states were projected on to these two distances and contour maps plotted for the Cα-Cα distances of R1023.50-E2286.30 and R1023.50-Y2887.53. The numbers 1, 2, and 3 in the figures correspond to the Cα-Cα distances in the crystal structures of inactive (PDB: 3PWH, number 1), active-intermediate (PDB: 2YDV, number 2), and the mini-Gs-bound fully active state of A2AR (PDB: 5G53, number 3).
(B) Representative structures extracted from the most populated cluster of A2AR bound to the agonist NECA in the inactive state (RNECA), the active-intermediate state (R′NECA), and the fully active G protein-bound state (R∗·GNECA). The R∗·G−NECA state is a metastable state observed upon MD simulation of the receptor after removal of the G protein. The color scheme ranges from red to blue, with blue indicating low flexibility and red high flexibility. The flexibility is quantified by the B factor calculated from root-mean-square fluctuation in Å.