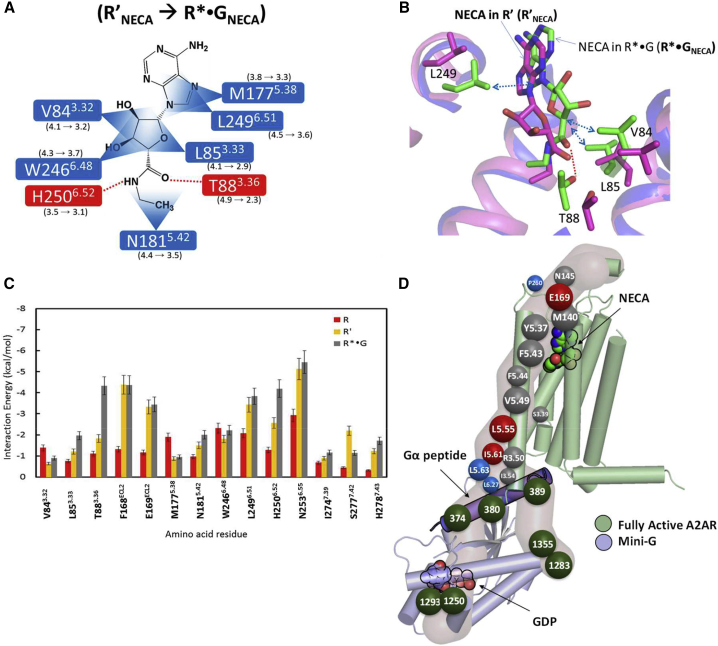
Figure 7.
The Effect of G Protein Coupling in Increasing the Ligand Affinity Going from the Active-Intermediate R′ State to G Protein-Bound Fully Active (R∗·G) State.
(A) The ligand-receptor contacts that showing over 20% increase in population between R′NECA and R∗·GNECA are shown. The numbers shown near each contact is the contraction in the average distance in each of these contacts going from R′NECA to R∗·GNECA.
(B) Representative structures of NECA binding site in R′NECA (pink) and R∗·GNECA (green) states with the residues that show significant contraction of ligand-residue distances in (A).
(C) The non-bond interaction energy (kcal/mol) between agonist NECA and the residues in the ligand binding site A2AR in the inactive state (R, red), active-intermediate state (R′, orange) and fully active state (R∗·G, black).
(D) The residues shown by their Ballesteros-Weinstein numbering scheme located in the allosteric communication pipeline from the EC region connecting the G protein-coupling residues via the ligand binding site. The size of the sphere is proportional to their strength contribution to the allosteric pipeline. Residues shown in gray spheres show reduced affinity for agonist when mutated to alanine, and those shown in blue spheres have an increased affinity for agonist, and the maroon sphere residues show less than 10% change in ligand binding upon mutation to alanine compared with the wild-type. The allosteric communication residues to the nucleotide (shown as outline in the figure) binding site in the G protein are shown in green spheres. The G protein numbering is taken from the PDB structure of A2AR with mini-Gs bound (PDB: 5G53). The A2AR receptor is shown in green and mini-Gs is in light blue.