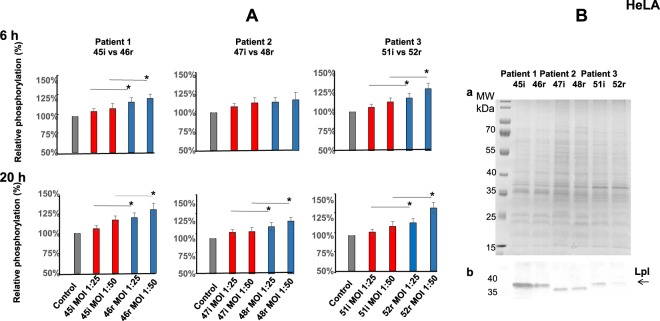
Figure 6.
S. aureus recurrent isolates induce stronger DNA damage in HeLa cells and express a lower amount of Lpls than initial acute isolates. (A) HeLa cells were exposed for 2 h to tree couples of isolates (MOI 1:25 and 50) that have been recovered from three patients (P1, P2, P3) at the time of the initial (45i, 47i, 51i, red) and relapsing (46r, 48r, 52r, blue) infection from P1, P2, P3 correspondently. At 6 h and 20 h post-infection phosphorylated γH2AX was quantified by immunofluorescence analysis using High Content Screening approach as described in Material and Methods. DNA and ɣH2AX-immunofluorescence were visualized using a Cellomics ArrayScan VTI HCS Reader (Thermo Fisher) ImPACcell technologic platform. Ten high-definition images per well of a 96 multiwall plate were analyzed and an arbitrary immunofluorescence value was found for each nucleus. Results are expressed as a percentage of nucleus labelled with ɣH2AX. The relative phosphorylation of the control cells was considered as 100%. Percent of the relative phosphorylation of samples was calculated as fold changes over the control and multiplied by 100. Data are presented as mean ± SD from three independent experiments. P-values, 0.05 (*) were considered to be significant. (B) S. aureus strains isolated from three patients (P1, P2, P3) with initial (45i, 47i, 51i) and recurrent (46r, 48r, 52r) BJI from P1, P2, P3 correspondently were maintained as described. Lpls enriched fractions were prepared as indicated in Material and Methods. TritonX114 lipoprotein-enriched fractions, prepared from 6 clinical isolates, were separated on 12% SDS-PAGE: (a) Gel was stained with Coomassie blue (b) The Western blot analysis was performed using anti-Lpl1-his antibody which we developed. Molecular Weight markers are presented at the left side of SDS-PAGE gel and membrane. The arrow indicates S. aureus LpLs.