FIG 5.
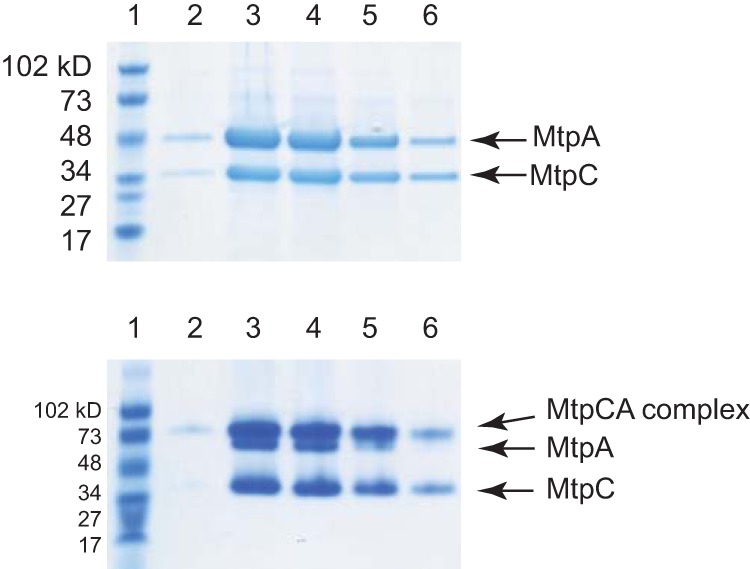
MtpA and MtpC form a complex. Cell-free extract from WWM973 was subjected to affinity purification. The eluted proteins were then separated by electrophoresis and stained with Coomassie blue. (Top) A denaturing SDS-PAGE gel (4% to 20%) was loaded with fractions from different steps of protein purification. Lane 1, protein standards; lanes 2 to 6, elution fractions. (Bottom) The same fractions were loaded on a nondenaturing PAGE gel (4% to 20%). At the pH of running buffer (pH 8.5), both MtpC (pI = 4.04) and MtpA (pI = 4.52) are negatively charged.
