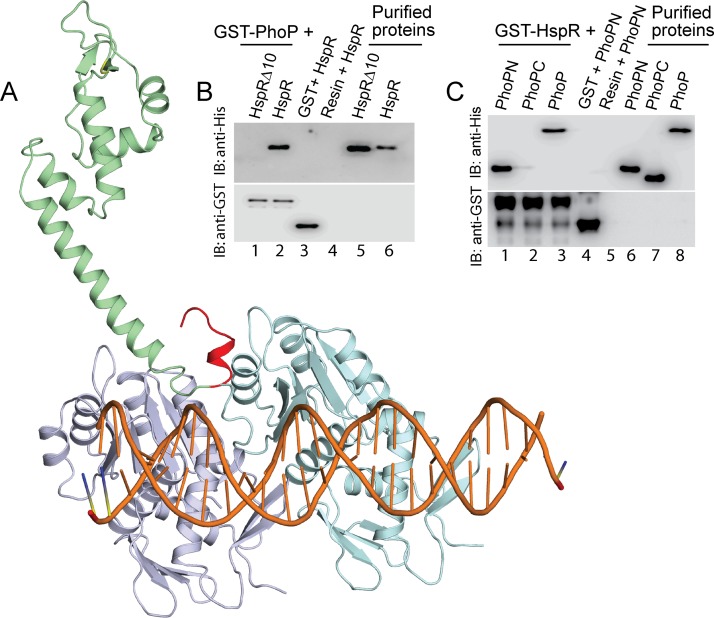
FIG 4.
Docking of HspR structure on PhoP-DNA complex. (A) The docking of HspR structural model on PhoP-DNA complex utilized a submission of the respective structural coordinates to the docking server ZDock (20) as detailed in Results. (B) To examine the role of the C-terminal 10 residues of HspR (shown in red in panel A) in PhoP-HspR interactions, crude lysates of cells expressing His6-tagged HspRΔ10 were incubated with glutathione-Sepharose, previously immobilized with GST-PhoP. Fractions of bound proteins (lane 1) were analyzed by Western blotting using anti-His (top) or anti-GST (bottom) antibody. Control sets include glutathione-Sepharose immobilized with GST alone (lane 3) or the resin alone (lane 4); lanes 5 and 6 resolve purified HspRΔ10 and HspR, respectively. (C) In vitro interactions of HspR and indicated PhoP domains (PhoPN and PhoPC) were investigated by incubating crude cell lysates of His6-tagged PhoP domains with glutathione-Sepharose previously immobilized with GST-HspR. The results suggest that the PhoPN, and not PhoPC, retains the ability to interact with HspR (see Results for more details). Note that PhoP N and PhoP C domain constructs, used in this study, were previously shown to be fully functional on their own (21).