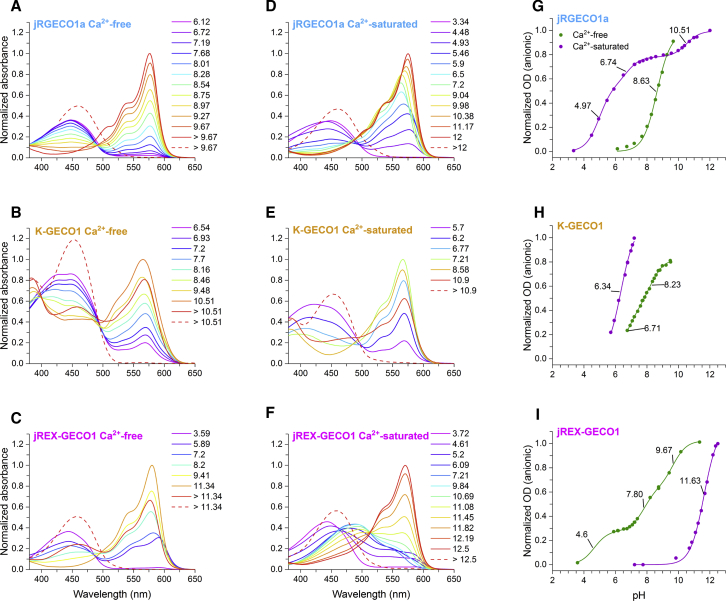
Figure 4.
Absorbance pH titrations and shifts in pKa are starkly different between classes and between their Ca2+-free and Ca2+-saturated states. Titrations from neutral to acidic pH and neutral to alkaline pH were done separately and then combined. The figures are normalized to the observed maximal absorbance of the anionic form. (A–F) Representative spectra from absorbance pH titrations are shown, measured in either Ca2+-free or Ca2+-saturated buffer as noted. Each spectrum was measured at the pH value designated in the legends. The final spectrum (red dashed line) belongs to the denatured chromophore. (G–I) Apparent pKa curves are shown, showing the OD of the anionic form as a function of pH. The pKa values are indicated on the fitted curve. (H) Only part of the K-GECO1 titrations could be fitted because of protein precipitation at pH values below those displayed and early denaturation of the anionic form at higher pH values.