Abstract
Breast cancer in men is rare and only about 390 men in the UK are diagnosed with breast cancer each year with an incidence rate in the UK of 1.5 cases per 100 000 men. In addition, the increased use of radiotherapy for management of breast cancer has led to a reported increase of radiation induced angiosarcomas (RIAS) with an incidence of 0.05–0.3%. Here we report a unique and extremely rare case of RIAS of breast in a male patient. To our knowledge this is the only case in the literature of a radiation induced angiosarcoma of the breast in a male.
INTRODUCTION
Breast cancer in men is rare and only about 390 men in the UK are diagnosed with breast cancer each year with an incidence rate in the UK of 1.5 cases per 100 000 men [1, 2].
The management of breast cancer in male patients is the same as the one in females and therefore adjuvant radiotherapy is very commonly used. Unfortunately, the increased use of radiotherapy for management of breast cancer has led to a reported increase of radiation induced angiosarcomas (RIAS) with an incidence of 0.05–0.3% [3–5]. Here we report a unique and extremely rare case of RIAS of breast in a male patient.
CASE REPORT
A 72-year-old male was diagnosed with left breast invasive ductal carcinoma (tumor of 11 mm, stage I (T1N0M0), grade3, estrogen receptor (ER) and progesterone receptor (PR) positive, Ki-67 8%, EGFR and Her-2 negative) and underwent left breast mastectomy and lymph node axillary dissection.
His past medical history included atrial fibrillation, hypertension, hypercholesterolemia, glaucoma and he had a strong family history of breast carcinoma.
He received 20 days of adjuvant radiotherapy treatment and five years of endocrine adjuvant treatment of his chest wall with single field modality technique and a total dose of 4005 cGy D-Max in 15 fractions.
Six years after completion of adjuvant radiotherapy treatment for his breast cancer, the patient developed multiple purpuric nodules below and very close to the mastectomy scar and a punch biopsy revealed radiation induced angiosarcoma. A CT scan of his chest, abdomen and pelvis had shown no evidence of distant metastases. He underwent a wide resection of the mastectomy scar down to the ribs and the defect was reconstructed with pedicled latissimus dorsi flap in combination with a V-Y fashion adipocutaneous advancement flap from his abdomen (Fig. 1).
Figure 1:
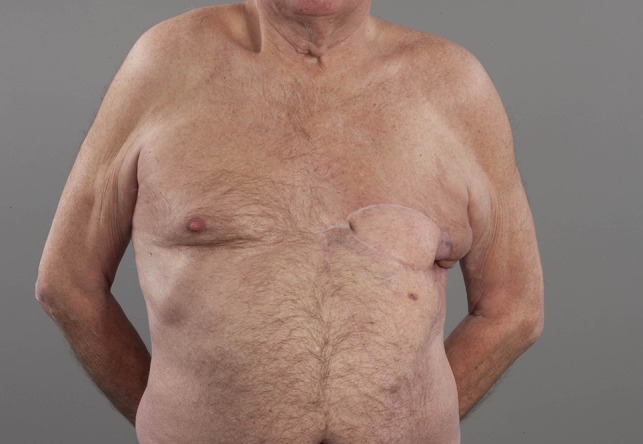
Patient in upright position. Left chest wall reconstruction with pedicled latissimus dorsi flap in combination with a V-Y fashion adipocutaneous advancement flap from his abdomen after resection of RIAS of his breast.
The histology report showed a multifocal grade 3 angiosarcoma (pT2a, pN0) involving the dermis and subcutaneous tissue composed of inter-anastomosing vascular channels lined by atypical endothelial cells. The nearest peripheral margin appeared to be approximately 10 mm and the deep margin from the tumor was documented as very close but free of tumor infiltration.
The patient had three more surgical treatments and one treatment with electrochemotherapy (Bleomycin 34 000 IU) with partial response to control local disease recurrence. The local disease-free interval was 8,5,7 and 3 months period between these treatments.
Unfortunately, he rapidly developed widespread disease over his chest wall that was not amenable to surgical treatment, nor further session of electrochemotherapy (Fig. 2).
Figure 2:
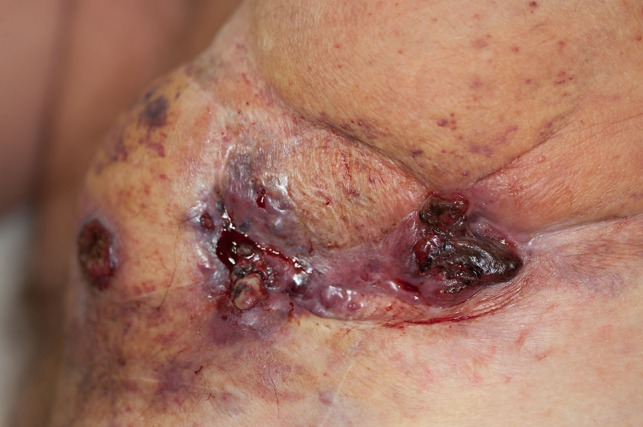
Patient in upright left lateral position. Rapidly developed wide spread disease over his left anterior and lateral chest wall not amenable to surgical treatment and electrochemotherapy. At this point chemotherapy treatment commenced with Paclitaxel.
After a long discussion with the patient and a multidisciplinary team consensus, a decision was made for further management with adjuvant chemotherapy. The proposed treatment by the medical oncology team was full dose Paclitaxel on a weekly basis which was started 3 months after his last electrochemotherapy session. The full dose had seemed to show significant improvement in his chest wall disease but unfortunately this was too toxic for the patient to tolerate and therefore on week 4 the dose was decreased (Fig. 3). This reduced the side effects of Paclitaxel in full dose, including severe neutropenia and excessive fatigue. Disease progression was noted on the reduced dose of Paclitaxel treatment and therefore the further treatment was changed to Pazopanib 600 mg per day. However, soon after commencement, the development of hypertension and confusion necessitated cessation of treatment.
Figure 3:
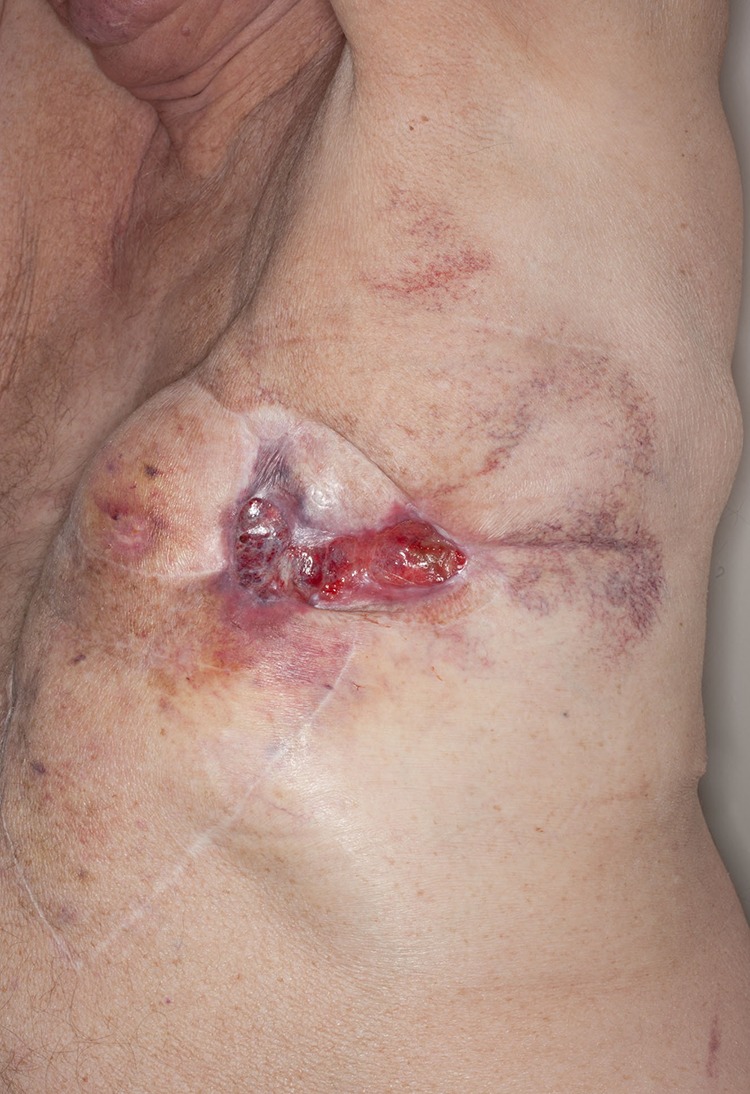
Patient in upright left lateral position. Significant control of his chest wall local disease progression with administration of full dose of Paclitaxel.
In the meantime, a chest X-ray revealed progressive interstitial thickening in the right mid and upper zone, confirming our clinical suspicion of rapid progression of disease on the patient’s chest wall.
At that stage, a decision was taken with the patient and his family to focus on symptom relief and best supportive care at home. The patient died 32 months after his first surgical treatment for the radiation induced breast angiosarcoma and eight years and five months following completing the adjuvant radiotherapy treatment for his breast cancer.
DISCUSSION
RIAS is relatively rare, with an estimated incidence at between 0.05 and 0.3% in women treated with radiotherapy. It is a well recognized late complication of adjuvant radiotherapy, with a latency period that varies from approximately five to ten years [6].
The incidence of RIAS does not appear to be influenced by the type of surgery performed (mastectomy or wide local excision) but there might be a potential interaction of radiotherapy and lymphoedema following treatment [4].
There may also be a dose response relationship between the dose of radiotherapy administrated and the incidence of RIAS with a minimum of 10 Gy associated with the development of the condition but usually associated with higher doses [4].
The 5-year survival rate of RIAS patients is reported in the literature in the range of 25–50% [7, 8]. The patient in our case died 32 months after his diagnosis for RIAS.
Furthermore, tumor grade is considered to be an important prognostic factor for RIAS. Patients with high grade tumor as in our case, have worse survival rate. Our patient had multiple episodes of local recurrences treated surgically and with electrochemotherapy but ultimately his disease progressed rapidly such that active treatment with curative intent was ceased.
He was subsequently referred to our medical oncology colleagues knowing that the role of chemotherapy for RIAS remains uncertain. An agent such as Paclitaxel has shown encouraging results in unresectable angiosarcomas but its dose-limiting toxic effects does not allow prolonged treatment for more than 6 months in most cases [9].
In our case the dose of paclitaxel initially had to be reduced and eventually stopped due to its toxic effect after four months of treatment. Subsequent attempts at disease control with a tyrosine kinase inhibitor, Pazopanib, had to be abandoned as well due to toxicities. His disease rapidly progressed, infiltrating deeper structures of his chest wall and the patient entered the supportive care pathway in order to ensure that he received the best possible care in the last period of his life.
Wide surgical resection with adequate clear histological margins remains the first line treatment for radiation-induced angiosarcoma.
To our knowledge this is the only case in the literature of a radiation induced angiosarcoma of the breast in a male.
CONFLICT OF INTEREST STATEMENT
None Declared.
REFERENCES
- 1.https://www.breastcanceruk.org.uk/science-and-research/background-briefings/male-breast-cancer/ https://www.breastcanceruk.org.uk/science-and-research/background-briefings/male-breast-cancer/
- 2.https://www.cancerresearchuk.org/about-cancer/breast-cancer/stages-types-grades/types/male-breast-cancer https://www.cancerresearchuk.org/about-cancer/breast-cancer/stages-types-grades/types/male-breast-cancer
- 3. Monroe AT, Feigenberg SJ, Mendenhall NP. Angiosarcoma after breast-conserving therapy. Cancer 2003;97:1832–40. [DOI] [PubMed] [Google Scholar]
- 4. Sheth GR, Cranmer LD, Smith BD, Grasso-Lebeau L, Lang JE. Radiation-induced sarcoma of the breast: a systematic review. Oncologist 2012;17:405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torres KE, Ravi V, Kin K, Yi M, Guadagnolo BA, May CD, et al. . Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol 2013;20:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alves I, Marques JC. Radiation-induced angiosarcoma of the breast: a retrospective analysis of 15 years’ experience at an oncology center. Radiol Bras 2018;51:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mery CM, George S, Bertagnolli MM, Raut CP. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer 2009;115:4055–63. [DOI] [PubMed] [Google Scholar]
- 8. D’Angelo SP, Antonescu CR, Kuk D, Qin L, Moraco N, Carvajal RC, et al. . High-risk features in radiation-associated breast angiosarcomas. Br J Cancer 2013;109:2340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zemanova M. Clinical management of secondary angiosarcoma after breast conservation therapy. Rep Pract Oncol Radiother 2013;19:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


