Figure 8. Schematic representing of RhoE-mediated negative regulation of inflammation.
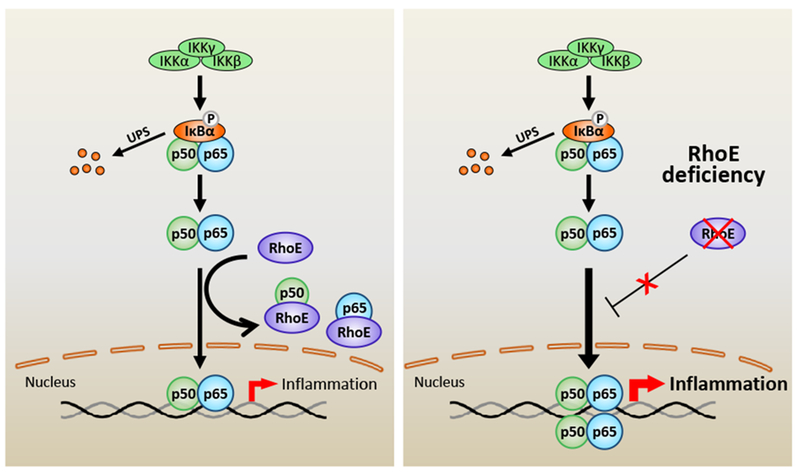
NF-κB is the pivot transcriptional regulator of inflammatory responses. Classical NF-κB pathway is primarily activated by IκB kinase (IKK)-mediated phosphorylation and degradation of IκBα. Activated p65/p50 complex is then translocated into nucleus to promote expression of target genes. RhoE functions as a novel NF-κB suppressor. RhoE physically binds to p65 and p50 individually in cytosol to inhibit their nuclear translocation. RhoE-p65 and RhoE-p50 interactions also impede formation of the p65/p50 heterodimer. Under RhoE deficiency condition, the inhibitory role of RhoE on NF-κB is diminished, which promotes the heterodimerization of p65 and p50, and the nuclear translocation of the p65/p50 complex, resulting in overactivated NF-κB and excessive inflammation.
