Abstract
While the reproductive benefits of sexual displays have been widely studied, we have relatively limited evidence of the fitness costs associated with most display traits. Insect cuticular hydrocarbon (CHC) profiles are sexually selected traits that also protect against desiccation. These two functions are thought to oppose each other, with investment in particular compounds believed to increase attractiveness at the expense of compounds that protect against water loss. We investigated this potential trade-off in a quantitative genetic framework using the Australian field cricket, Teleogryllus oceanicus. Several compounds were significantly genetically correlated with either attractiveness or desiccation resistance. Of these compounds, one was negatively genetically correlated with attractiveness but positively genetically correlated with desiccation resistance. Furthermore, scoring each individual's overall CHC profile for its level of attractiveness and desiccation resistance indicated a negative genetic correlation between these multivariate phenotypes. Together, our results provide evidence for a genetic trade-off between sexually and naturally selected functions of the CHC profile. We suggest that the production of an attractive CHC profile may be costly for males, but highlight the need for further work to support this finding experimentally. Genetic covariation between the CHC profile and attractiveness suggests that females can gain attractive sons through female choice.
Keywords: sexual signals, costs, cuticular hydrocarbon, desiccation, female choice, Teleogryllus oceanicus
1. Introduction
Increased male mating success through the evolution of elaborate secondary sexual traits is thought to come at a cost of decreased viability [1–3]. For example, sexual signals can attract not only mates but predators and parasitoids [4]. While being more attractive or producing more exaggerated sexual traits can increase mating success, it can also reduce lifespan [5,6]. Costs associated with sexual trait expression have important implications for the evolution of secondary sexual traits, providing a constraint to further elaboration [2], a mechanism to link sexual displays to individual quality [7,8] and an avenue for the maintenance of genetic variation [9,10]. Despite the importance of costs to our understanding of the evolution of secondary sexual traits, we still have a limited knowledge of the costs associated with many well-studied sexual displays [11].
The cuticular hydrocarbons (CHCs) of insects act as a sexual display in a number of species [12–20], but we have only a limited understanding of the viability costs (if any) of producing an attractive CHC profile. CHCs are long-chained compounds that both have a signalling function and provide a barrier to water loss across the cuticle [21]. Generally, a number of different compounds make up the CHC profile, and these compounds can vary in both their chain length and the presence or absence of methyl branches and double bonds [22]. Variation in the structure of CHC compounds is associated with variation in their physical properties, such that increasing chain length increases their melting temperature, which is decreased by double bonds and methyl branches [23]. Variation in the composition of insect CHC profiles, and consequently variation in melting temperatures, is thought to have important implications for both waterproofing and signalling functions. In particular, a CHC profile that displays a greater degree of melting at ecologically relevant temperatures should facilitate the detection of signalling compounds but have reduced waterproofing abilities. By contrast, a more solid CHC profile is likely to hinder the detection of these compounds but reduce water loss across the cuticle [24–26]. Using CHCs as a sexual display trait can therefore result in a viability cost, where investment in compounds that increase attractiveness decreases the waterproofing functionality of the CHC layer, and consequently the ability to withstand desiccation.
Despite the potential for the production of an attractive CHC profile to be costly in terms of desiccation resistance, little empirical evidence of such a trade-off exists. Most investigations of the association between CHCs and male mating success have found complex patterns of linear and nonlinear selection [27], with the association between the level of attractiveness of a CHC blend and its waterproofing properties unclear. Experimental evolution using the fruit fly, Drosophila simulans, found that a principal axis of CHC variation favoured under high temperatures only evolved in the absence of sexual selection [28]. However, as the effect of this particular blend of CHCs on the waterproofing properties of D. simulans is unknown [28], it is not possible to ascertain whether this evolutionary response reflects a trade-off between CHC attractiveness and desiccation resistance. Although the potential for trade-offs between attractive and protective CHC profiles certainly exists, increased investment in compounds with mid-range effects on the fluidity of the CHC layer could be favoured by both natural and sexual selection [29]. For example, a series of experiments found that one methyl-branched compound had positive effects on both mating success and desiccation resistance in male D. serrata [30]. The general lack of evidence that producing an attractive CHC profile results in a viability cost through decreased desiccation resistance, along with the possibility that natural and sexual selection may favour investment in the same CHCs, necessitates further investigation of this topic.
Testing for negative genetic correlations is a useful approach for addressing the question of whether or not the production of an attractive CHC profile comes at a cost of reduced desiccation resistance [9]. For example, provided that the necessary genetic variance exists, if investment in a particular compound increases attractiveness at the expense of reduced desiccation resistance, we would expect to see a positive genetic correlation between that compound and attractiveness, and a negative genetic correlation with desiccation resistance. Similarly, if the blend of CHCs that maximizes mating success differs from the blend that maximizes desiccation resistance, then a negative genetic correlation between the production of the different blends can reveal the trade-off between them. There is evidence that investment in longer-chained CHC compounds is positively genetically correlated with desiccation resistance in D. melanogaster [31], although whether this corresponds to a negative genetic correlation with attractiveness is not known. Similarly, the results from two artificial selection experiments indicate the existence of a genetic correlation between mating success and the blends of CHCs predicted to be attractive in male D. serrata, and these attractive blends of CHCs appear to be opposed by natural selection [32,33]. However, whether the opposing natural selection arises through desiccation associated viability selection is unknown. Although there is evidence for genetic correlations between CHCs and attractiveness, and between CHCs and desiccation resistance, ascribing desiccation related costs to an attractive CHC profile requires both to be measured in the one study. We sought to investigate this question by testing for genetic correlations between the CHC profile, attractiveness and desiccation resistance using the Australian field cricket, Teleogryllus oceanicus.
CHCs have been found to play a significant role in influencing male mating success in T. oceanicus. Ablation of female chemoreceptors reduces the likelihood they will mount a courting male [34], and a combination of linear and nonlinear selection exerted by female mate choice appears to be acting on male CHC profiles [15,35]. CHCs are heritable in T. oceanicus [36], and although the estimates are low, attractiveness is significantly repeatable [15], indicating that this fitness component may also be heritable. Among isolated populations of T. oceanicus introduced to the Hawaiian Islands there have been independent evolutionary origins of males unable to produce song, favoured by selection from the acoustically orienting parasitoid fly Ormia ochracea [37–39]. These ‘flatwing' males are no longer able to attract females using song, but they have an increased investment in relatively short-chained CHC compounds (C31 versus C33 and C35 compounds), potentially as a method for increasing attractiveness in the absence of song [40]. A common-garden study found that males derived from islands with higher precipitation also produced a greater proportion of relatively short-chain compounds, suggesting a role for both sexual and natural selection in shaping variation in CHC profiles [40]. Collectively, these findings raise the hypothesis that greater investment in shorter-chained compounds increases attractiveness, but that this comes at a cost of reduced desiccation resistance in T. oceanicus.
We used the quantitative genetic animal model [41–44] to test the hypothesis that greater investment in relatively shorter-chained CHCs will be positively genetically correlated with attractiveness, but that these positive genetic correlations will be mirrored by negative genetic correlations with desiccation resistance. The reverse relationship is expected to occur with increasing CHC chain length. Although intuitive, this hypothesis relies on a relatively simple relationship between CHC chain length and the fluidity/permeability of the CHC profile, and ignores how particular blends of CHCs may act together in influencing attractiveness and desiccation resistance. We therefore also use multivariate methods to generate univariate scores of attractiveness/desiccation resistance for each individual's CHC profile. If producing a more attractive CHC profile comes with a viability cost of reduced desiccation resistance, we would expect to see a negative genetic correlation between these scores.
2. Methods
(a). Breeding design and cricket husbandry
A multi-generation pedigreed breeding design was formed by first mating 15 wild-caught male T. oceanicus crickets each to three previously unmated females from an established laboratory stock population. To produce a second generation of crickets, female offspring from these families were then mated to the male offspring of 19 field-inseminated females that had been collected at the same location and time as the wild-caught males described above. Only one male offspring from each field-inseminated female was used and each of these was mated to three females taken from across different families within the breeding design. Some females did not produce offspring due to either death or infertility, resulting in some males having offspring with less than three females. Both the laboratory stock population and wild-caught crickets were sourced near Carnarvon, Western Australia, with the laboratory population being supplemented annually with wild-caught crickets. The breeding design produced offspring from a total of 85 mothers and 34 fathers across two generations, resulting in full-sibling, half-sibling, maternal cousin and uncle–nephew relations.
After mating, each female was housed separately and allowed to oviposit in moist cotton wool. At least two replicate groups of offspring from each female were housed in 15 l plastic containers, given egg cartons for shelter and free access to food (cat chow) and water. Freshwater and additional food if required were given weekly. Male crickets were removed from these containers at the penultimate instar and placed individually in plastic containers (7 × 7 × 5 cm) with free access to food and water. Upon adult eclosion, the container was cleaned and each cricket was given 190 ± 10 mg cat chow. Variation in resource acquisition [45,46] and the availability of surplus resources [9] can both mask trade-offs between life-history traits. For example, a previous study using T. oceanicus revealed a trade-off between immunity and sperm viability when crickets were fed a restricted diet, but not when they were fed ad libitum [47]. Adult crickets were therefore provided a restricted diet to increase the likelihood that trade-offs would be revealed. A preliminary study had found that the average total cat chow consumed by an individual male cricket over 12 days was 356 mg (n = 21, s.e. = 24 mg). The diet we provided therefore represented 53% of the average consumption over this time period. Crickets were kept in a controlled temperature room on a 12 : 12 h light : dark cycle at 26°C for 12 ± 2 days before being assayed and/or freeze killed for later measurement.
(b). Attractiveness and desiccation resistance
To measure attractiveness, at 12 ± 2 days post-eclosion, male offspring were placed in a clean container with a single stock female in a no-choice mating trial. Following commencement of the male courtship song, pairs were observed for 5 min and the time taken for a female to mount the male and receive a spermatophore was recorded. Those males that were not mounted by 2 min were assigned a status of unmated. Two minutes was selected because a preliminary survival analysis revealed that 50% of males were successfully mounted by the female two minutes after the commencement of courtship (males not mated within the 5 min of observation were assigned a census time of 5 min for the preliminary survival analysis). Measuring attractiveness (mated = 1 versus unmated = 0) as a binary trait was necessary to ensure that all individuals for which we assayed attractiveness could be incorporated in the quantitative genetic analyses, including those that were not mated within the observation period. Females used in the mating assay were between 7 and 9 days old. These females had been kept in female-only groups until the day before the mating trial, at which time they were housed individually with a single stock male for eight hours to ensure prior mating experience, as this has been shown to increase the level of female choosiness in another species of cricket [48]. All observations took place at 26°C under red light to minimize disturbance of the crickets. Following the mate choice trial, male crickets were returned to their original container to be used in a desiccation assay the following day.
To measure desiccation resistance, each cricket was placed in a new container that held 30 g of desiccant (silica gel, SiO2), separated from the cricket by a plastic barrier, and placed in an incubator set at 30°C for 18 h. After this time crickets were checked every hour for a further 18 h and their time of death recorded. The majority (88.64%) of crickets died between 19 and 36 h. However, some crickets were found dead at the first check (6.58%) or still alive after 36 h (4.78%). As with our attractiveness data, it was, therefore, necessary to measure desiccation resistance as a binary trait to ensure that data from all individuals could be included in the quantitative genetic analyses, including those for which we did not observe their time of death. A survival analysis revealed that 50% of the crickets died within 28 h in the incubator (males still alive at the end of the observation period were assigned a census time of 36 h for this analysis). We therefore assigned those individuals still alive at 28 h desiccation resistant (1), with the remainder assigned as desiccation susceptible (0). Crickets were frozen immediately after death or at the end of the trial if they were still alive. Both assays (attractiveness and desiccation resistance) were conducted blind to an individual's pedigree links.
(c). Cuticular hydrocarbons
For CHC extraction, crickets were immersed for 5 min in 5 ml of hexane containing 0.02 g l−1 of n-dotriacontane as an internal standard. One microlitre of the sample was injected using the splitless mode into a Shimadzu QP2010 gas chromatography–mass spectrometry (GC-MS) machine fitted with a Stabilwax column (30 m × 250 µm × 0.10 µm). The initial column temperature was set at 40°C for 1 min and then increased at 20°C per minute until reaching 250°C for 20 min. The compounds were visualized as peaks with the area under each peak representing the amount of the compound. Peaks were integrated using Shimadzu GCMSsolution software (v. 4.41) and matched to compounds previously identified in this species. To control for variation in the amount of solution injected across samples, all peak areas were divided by the area of the internal standard. For this study, we restricted our analyses to the seven compounds of greatest abundance which collectively represent over 80% of the total CHC abundance.
We measured the CHC compounds of two sets of individuals. The first set comprised a haphazardly selected subsample of individuals from across the different families for which we also had both attractiveness and desiccation resistance data. To minimize variation in the CHC profile which may result from differences in the time between death and freezing, we only measured the CHCs of individuals whose time of death we observed (that is, we did not measure the CHCs of individuals that were found dead at the first check in the desiccation chamber, or that were still alive at the end of the trial). Measuring the CHCs of these individuals allowed us to derive an estimate of the blends of CHCs predicted to have the strongest association with attractiveness and desiccation resistance. To do so we used multiple regression [49] with either attractiveness or desiccation resistance as the response variable and the seven CHC compounds as explanatory variables. Peak areas were scaled to a mean of zero and a standard deviation of one prior to the regression, and the attractiveness/desiccation resistance measures were scaled by their mean [49]. Scaling fitness components by their means allows the returned model coefficients to be interpreted as selection gradients [49], facilitating the comparison of our results with other studies examining multivariate selection on CHCs. We note that mean scaling has no effect on the estimated genetic correlations. We included the inverse of the relatedness matrix as a random effect in these models to control for the non-independence of individuals due to their relatedness. The returned vectors of partial regression coefficients from these models are presented in table 1.
Table 1.
Modelling cuticular hydrocarbon (CHC) attractiveness and desiccation resistance. Coefficients (β) from multiple regression models with relative attractiveness or desiccation resistance as the response variable and the seven highest abundance CHC compounds as explanatory variables. Standard errors are given in parentheses.
| attractiveness | desiccation resistance | |
|---|---|---|
| 4-MeC30 | −0.082 (0.100) | −0.023 (0.095) |
| C31:1 | 0.336 (0.112) | −0.083 (0.106) |
| C31:2 | −0.071 (0.113) | −0.003 (0.110) |
| 4-MeC33 | −0.153 (0.114) | 0.042 (0.108) |
| C33:1 | 0.020 (0.114) | 0.103 (0.107) |
| C33:2 | 0.258 (0.109) | 0.029 (0.104) |
| C33:2 | −0.272 (0.139) | −0.002 (0.132) |
The second set of individuals comprised of crickets that had not undergone a mating or desiccation trial but were instead frozen at 12 days of age. CHCs are known to respond to changes in environmental and social conditions in several species [50,51] including T. oceanicus [52–54]. Using individuals that had not been exposed to a female or the desiccating environment allowed us to exclude potential effects of these factors on the CHC profile in our tests for genetic correlations. We therefore used the individual CHC compound data from this set of crickets in our quantitative genetic analyses. We also scored these individuals for their predicted CHC profile attractiveness (Att-CHCβ) and desiccation resistance (Des-CHCβ) by applying the partial regression coefficients derived from the first set of individuals to the scaled CHC data of this second set of crickets [55–57]. This provided a univariate measure of an individual's multivariate CHC profile attractiveness and desiccation resistance to be used in the quantitative genetic analyses.
(d). Quantitative genetic analyses
As a preliminary analysis, we first fit univariate ‘animal’ models in a REML framework incorporating information from the pedigree to estimate the additive genetic variance for each trait [43]. Full results of these models are presented in electronic supplementary material, table S1. Significant additive genetic variance (VA) was found for all traits. Estimates of h2 can be inflated by non-modelled maternal effects [58]; however, we found no evidence for significant maternal effects for any trait in this study (electronic supplementary material, table S1). We therefore chose not to include maternal identity as an additional random effect in any of the following models.
It is possible that the two sets of conditions experienced by the crickets for which we measured CHCs resulted in differences in CHC expression. Recall that the partial regression coefficients were derived from individuals that had been assayed for attractiveness and desiccation resistance, and then used to estimate the CHCβ traits of individuals that had not been exposed to either a female or desiccating environment. Changes to the CHC profile induced by these environmental differences could be a source of error in our estimates of the CHCβ traits. To investigate this, we employed a character state genotype by environment (G × E) approach by treating each CHC compound measured in the two environments as separate traits, and testing for genetic correlations between them [59]. We tested the significance of each genetic correlation (rG) by comparing the unconstrained bivariate animal model to one where the rG was constrained to zero using log-likelihood ratio tests (LLRT) with 1 d.f. Tests for significant G × E were conducted in a similar manner but constraining the rG to approximately 1 (0.99). The results of these tests are presented in electronic supplementary material, table S2. Each compound was significantly genetically correlated across the two environments and there was no statistical support for a G × E for any compound. An additional source of potential error in the CHCβ traits is the error associated with each estimated partial regression coefficient used in their calculation. However, a portion of the error associated with each partial regression coefficient will be caused by correlations among the CHC compounds, and this portion of the error will ‘cancel out' when scoring individuals for the CHCβ traits [60]. Nevertheless, to investigate how well the CHCβ traits predicted an individual's CHC profile attractiveness or desiccation resistance, we tested for genetic correlations between each CHCβ trait and their respective fitness component in a multivariate model that included all four traits (both CHCβ traits, attractiveness and desiccation resistance—see below). Both genetic correlations were positive and statistically significant (table 2). This result, coupled with the absence of significant G × E interactions for any compound, indicates that the CHCβ traits do at least partly reflect the multivariate attractiveness and desiccation resistance of the CHC profiles used in the quantitative genetic analyses. We return to this topic in the Discussion.
Table 2.
Results of the multivariate animal model testing for genetic correlations between attractiveness, desiccation resistance, Att-CHCβ and Des-CHCβ. Genetic correlations are given in the lower triangle and their associated p-values are given in the upper triangle. Standard errors are given in parentheses. p-values < 0.05 are shown in italics, along with their corresponding genetic correlation.
| attractiveness | desiccation resistance | Att-CHCβ | Des-CHCβ | |
|---|---|---|---|---|
| attractiveness | 0.737 | 0.029 | 0.042 | |
| desiccation resistance | 0.125 (0.365) | 0.907 | 0.009 | |
| Att-CHCβ | 0.701 (0.305) | 0.026 (0.221) | <0.001 | |
| Des-CHCβ | −0.694 (0.325) | 0.579 (0.187) | −0.655 (0.112) |
We tested for a trade-off between the attractiveness and desiccation resistance of T. oceanicus CHC profiles in two ways. First, we tested for a trade-off between the desiccation resistance and attractiveness functions of T. oceanicus CHC profiles by estimating the genetic correlation between Att-CHCβ, Des-CHCβ, attractiveness and desiccation resistance in a multivariate animal model. Testing for a negative genetic correlation between Att-CHCβ and Des-CHCβ allowed us to assess whether producing the blend of CHCs predicted to maximize attractiveness trades off genetically with the production of a more desiccant resistant CHC profile. Similarly, if investment in the blend of compounds predicted to increase attractiveness (Att-CHCβ) reduces overall desiccation resistance, we predict a negative genetic correlation between these two traits. Finally, we used this multivariate model to estimate the genetic correlation between our measures of attractiveness and desiccation resistance to test whether overall attractiveness trades off with overall desiccation resistance. We used LLRTs with 1 d.f. to test the significance of each genetic correlation by comparing the unconstrained model to one where the genetic correlation under test was constrained to zero.
We next used bivariate animal models to estimate the genetic correlations between each CHC compound and the measures of attractiveness and desiccation resistance. These models allowed as to explicitly test the hypothesis that investment in particular compounds, such as those with shorter chain lengths, increase attractiveness at the expense of decreased desiccation resistance, providing a complementary analysis to the multivariate model described above. Where greater investment in a compound simultaneously increases attractiveness and decreases desiccation resistance, we would expect to see a positive genetic correlation between the compound and attractiveness, and a negative genetic correlation between the compound and desiccation resistance.
All analyses were performed in R [61] using ASReml-R for fitting the multiple regression and quantitative genetic analyses [62], OIsurv for the survival analyses [63], ggplot2 for plotting [64] and the boot package for bootstrapping [65]. We note that inference based on penalised quasi-likelihood when fitting generalized linear mixed models in ASReml-R may not provide accurate results [62]. We therefore treated our binary measures of attractiveness and desiccation resistance as Gaussian traits in all animal model analyses, as this method should provide unbiased estimates of the genetic correlations [66–68]. To examine the validity of this approach, we conducted additional analyses where we estimated the genetic correlations between our two fitness measures and each of the CHC traits using weighted regressions of sire family means. Although this method will likely underestimate the magnitude of the genetic correlation, it should provide an unbiased indication of its sign (see [69] for further details and for a comparison with the animal model). As this analysis occurs at the level of sire family means, binary traits at an individual level can satisfy the requirements for fitting a linear model. In our case, an inspection of model diagnostic plots indicated all regressions using sire family means returned normally distributed model residuals. Bootstrapping at the sire family level was used for standard error estimation and inference in this instance.
3. Results
We obtained attractiveness data from 736 offspring (mean ± s.e. offspring per full-sibling family = 8.66 ± 0.39), and desiccation resistance data from 669 offspring (mean ± s.e. offspring per full-sibling family = 7.87 ± 0.36). The lower number for desiccation resistance was a result of space constraints in the desiccating incubator. We measured the CHCs of 252 offspring that had been measured for both attractiveness and desiccation resistance (mean ± s.e. offspring per full-sibling family = 2.96 ± 0.10). Finally, we measured the CHCs of 256 offspring that had not been assayed for attractiveness or desiccation resistance (mean ± s.e. offspring per full-sibling family = 3.01 ± 0.08).
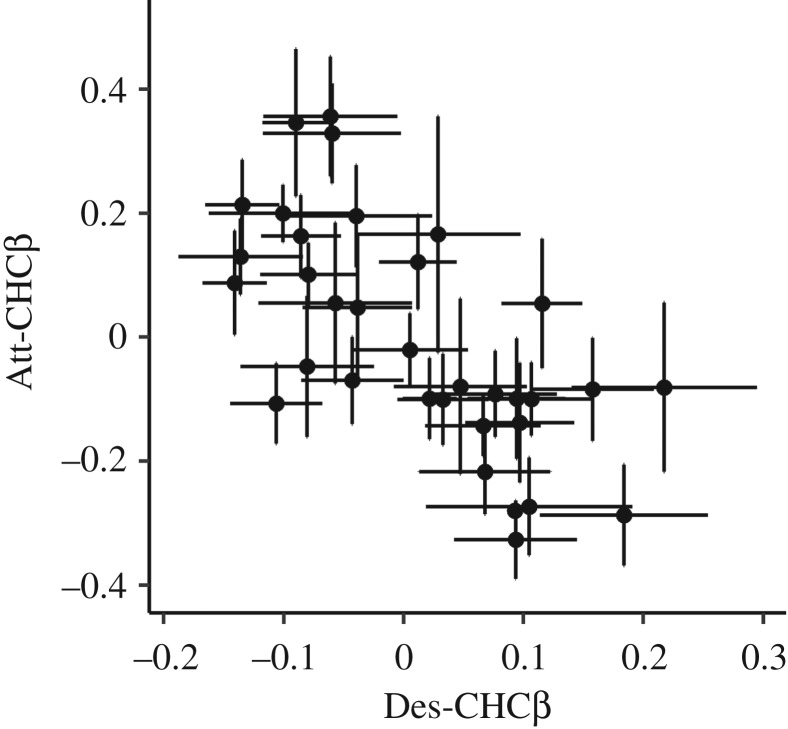
Table 2 presents the genetic correlations from the multivariate animal model that included attractiveness, desiccation resistance, Att-CHCβ and Des-CHCβ. As mentioned above, both CHCβ traits were significantly positively genetically correlated with their respective fitness measure (table 2). Furthermore, Des-CHCβ was negatively genetically correlated with attractiveness (table 2). Examining the vector of partial regression coefficients used to calculate Des-CHCβ (table 1) revealed that this relationship was largely driven by a contrast in the two alkenes (C33:1 and C31:1). That is, producing a CHC blend that comprised greater investment in C33:1 and reduced investment in C31:1 was associated with increased desiccation resistance and reduced attractiveness. However, investing in the blend of compounds predicted to maximize attractiveness (Att-CHCβ) was not negatively genetically correlated with overall desiccation resistance (table 2). Inspecting the vector of partial regression coefficients used to calculate Att-CHCβ (table 1) revealed that the compound with the strongest association with desiccation resistance (C33:1) had the least influence on an individual's Att-CHCβ score, potentially explaining the lack of association between this trait and desiccation resistance. There was a negative genetic correlation between the predicted attractiveness of the CHC profile (Att-CHCβ) and its predicted ability to withstand desiccation (table 2). The family means for the two CHCβ traits are plotted in figure 1 to visualize the negative genetic correlation between these traits. Despite the presence of a trade-off in the CHC profile, there was no evidence to suggest that overall attractiveness trades off directly with desiccation resistance (table 2).
Figure 1.
Plot of sire family means to visualize the negative genetic correlations between predicted cuticular hydrocarbon (CHC) profile attractiveness (Att-CHCβ) and predicted CHC profile desiccation resistance (Des-CHCβ).
Table 3 presents the results of tests for genetic correlations (rG) between the CHC compounds and attractiveness and desiccation resistance. The model estimating the genetic correlation between one CHC compound (4-MeC33) with attractiveness did not converge. We therefore used a weighted regression of sire family means to estimate this particular genetic correlation. Three of the relatively longer-chained CHC compounds were significantly negatively genetically correlated with attractiveness. Two of the longer-chained compounds were significantly positively genetically correlated with desiccation resistance. Of these compounds, one alkene (C33:1) was negatively genetically correlated with attractiveness but positively genetically correlated with desiccation resistance.
Table 3.
Results of the bivariate animal models testing for genetic correlations (rG) between each of the cuticular hydrocarbon (CHC) compounds with the measures of attractiveness and desiccation resistance. Standard errors are given in parentheses. p-values < 0.05 are shown in italics.
| attractiveness |
desiccation resistance |
|||
|---|---|---|---|---|
| rG | p-value | rG | p-value | |
| 4-MeC30 | 0.252 (0.367) | 0.491 | 0.137 (0.277) | 0.627 |
| C31:1 | 0.231 (0.333) | 0.479 | −0.315 (0.233) | 0.195 |
| C31:2 | 0.331 (0.368) | 0.357 | −0.082 (0.261) | 0.751 |
| 4-MeC33a | −0.464 (0.173) | 0.020 | 0.443 (0.254) | 0.112 |
| C33:1 | −0.931 (0.359) | 0.023 | 0.594 (0.252) | 0.046 |
| C33:2 | −0.283 (0.335) | 0.402 | 0.542 (0.233) | 0.042 |
| C33:2 | −0.693 (0.296) | 0.037 | −0.137 (0.267) | 0.618 |
aThe genetic correlation between 4-MeC33 and attractiveness was estimated using a regression of sire family means with bootstrapping at the family level for standard error estimation and inference.
As expected, using a weighted regression of sire family means gave lower estimates of genetic correlations (electronic supplementary material, tables S3 and S4) compared to those returned by the animal models (tables 2 and 3). With the exception of the genetic correlation between C33:1 and desiccation resistance, all genetic correlations that were found to be statistically significant using the animal model (tables 2 and 3) were also found to be statistically significant using a weighted regression of family means (electronic supplementary material, tables S3 and S4).
4. Discussion
We tested whether producing an attractive CHC profile results in a viability cost of increased susceptibility to desiccation for male Australian field crickets, T. oceanicus. Consistent with longer-chained CHCs reducing the fluidity of the CHC profile and its detectability by female chemoreceptors, the genetic correlation between investment in three relatively longer-chained compounds and attractiveness was negative. Similarly, consistent with longer-chained CHCs reducing water loss across the cuticle, two of the relatively longer-chained CHCs were positively genetically correlated with desiccation resistance. One CHC compound displayed evidence for an evolutionarily significant trade-off between the attractiveness and desiccation resistant functions of CHC compounds, with the genetic correlations between this compound and the two fitness traits being of opposite sign. Further evidence for a trade-off was revealed by modelling each individual's multivariate CHC profile. Using these predictive scores revealed that producing the CHC profile that maximized attractiveness was negatively genetically correlated with producing the CHC profile that maximized desiccation resistance.
Despite evidence of a trade-off between the attractiveness and desiccation resistance functions of the CHC profile, we found no evidence for a trade-off between overall attractiveness and desiccation resistance, with the genetic correlation between these traits weakly positive and not significantly different from zero. The CHC profile of crickets is only one of multiple traits likely to influence attractiveness and desiccation resistance. For example, variation in male courtship song is also under strong selection from female choice [35]. It is, therefore, possible that variation in unmeasured traits could have contributed to a positive covariation between attractiveness and desiccation resistance. Our results indicate that producing a desiccation resistant CHC profile reduces an individual's attractiveness, but that this negative effect could be overwhelmed by the positive effects of other traits. We suggest that further investigations that include additional life-history traits will be useful to gain a more comprehensive understanding of the association between attractiveness and desiccation resistance.
Water loss across the cuticle has been found to be an important contributor to total water loss in at least some insects, and CHCs provide an important barrier to water loss [21]. However, the phenotypic (represented by the partial regression coefficients), and to some extent the genetic, associations between desiccation resistance and the CHC profile were generally smaller than those between the CHC profile and attractiveness. There are a number of potential explanations for this result. Although desiccation resistance is a direct viability cost that affects fitness [11], CHCs affect this fitness component only indirectly through a reduction in cuticular water loss. Other factors might also affect an individual's ability to withstand desiccation. For example, traits such as the degree of melanisation can influence cuticular water loss [70,71], and increased resistance to desiccation in flies artificially selected for desiccation resistance is associated with an increase in carbohydrate as well as water content [72–74]. Variation in the CHC profile may therefore have a large effect on rates of cuticular water loss in T. oceanicus, but the effect on overall desiccation resistance could be obscured to some degree by other traits. Other studies have failed to find the predicted association between the rate of cuticular water loss and the physical properties of the CHC profile [25,75]. Gibbs [24] suggested that these findings may reflect localized variation in CHCs across the insect cuticle, which will be obscured when the body-wide CHC profile is examined. Such regional variation has been found in other insects [76,77], and if it occurs in T. oceanicus, our measure of the body-wide CHC profile may have prevented the detection of some variation relevant to desiccation resistance. Finally, our measure of desiccation resistance (probability of surviving) is likely to underestimate the costs of desiccation imposed from an attractive CHC profile. For example, crickets that produced an attractive CHC profile could have suffered non-lethal physiological costs of water loss in the desiccating environment, such as the ability to produce ejaculates or to transport oxygen or nutrients through the haemocoel. All, some or none of the above-mentioned factors could explain the weaker association between desiccation resistance and variation in the CHC profile than was initially hypothesized. We suggest that these possibilities provide exciting avenues for future research, particularly in the context of the interaction between natural and sexual selection.
Our finding of a negative genetic correlation between producing a CHC profile predicted to be attractive and one that is predicted to protect against desiccation should be interpreted with some caution. Multiple regression is a statistical technique that allows for the estimation of the effect of a trait on fitness, independent of the effects of the other traits included in the analysis [49]. An advantage of this form of analysis is the avoidance of assigning an impact of a trait on fitness when this is simply due to its correlation with other traits that have a fitness effect. The disadvantage of multiple regression is that the estimated associations with fitness may be moderated by the effect of traits not included in the analysis [49,78]. As our predictive models of CHC attractiveness/desiccation resistance were based on multiple regression analyses, it is possible that our finding of a negative genetic correlation between producing a CHC profile that was predicted to be attractive versus one predicted to be desiccation resistant was influenced by the effect of traits not included in the analysis. Nevertheless, results from multiple regression analyses have proven fruitful in CHC research. For example, two artificial selection experiments that selected upon the vector of male CHC attractiveness using D. serrata found an evolutionary increase in male attractiveness [32,33]. Similar studies will be required using T. oceanicus to provide further support for our finding of a trade-off between the blend of CHCs that make a male attractive and the blend that make him desiccation resistant. We emphasize that while the evidence we provide is supportive of a cost for producing an attractive CHC profile, it necessarily demands further experimental work.
The primary role of CHCs has been viewed as one of protecting the insect against desiccation, with signalling functions a subsequent evolutionary innovation [22]. Even if waterproofing is the primary function of CHCs, this is unlikely to explain the complex composition of insect CHC profiles, which under a purely waterproofing scenario could simply comprise of long straight-chained alkanes [79]. The results we present support the idea of the signalling function of CHCs being an important driver in shaping the complexity of the T. oceanicus CHC profile, with variation in the CHC profile strongly associated with variation in attractiveness. In particular, we found genetic correlations between male CHCs and attractiveness, indicating that females can use an assessment of the male CHC profile to gain attractive sons, which can have a strong effect on the evolution of sexual display traits [2]. Overall, our results suggest that producing an attractive CHC profile trades off with producing a CHC profile best suited to desiccation resistance. Our understanding of the viability costs of producing an attractive CHC profile is in its infancy, and we believe this topic provides an interesting area for future research.
Supplementary Material
Acknowledgements
We thank Fabian Rudin for assistance with breeding the crickets, Maxine Lovegrove and Karolina Berson for laboratory assistance, and Joseph Tomkins and Bruno Buzatto for statistical advice. The authors acknowledge the facilities, and the scientific and technical assistance of the Metabolomics Australia Facility at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments.
Ethics
Research was conducted according to University of Western Australia ethical guidelines.
Data accessibility
Data available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.r2j05j8 [80].
Authors' contributions
L.W.S., M.Z. and J.D.B. conceived the study. J.D.B. collected the data, performed the analyses and wrote the first draft of the manuscript. All authors contributed to the final manuscript and approved its contents.
Competing interests
We declare we have no competing interests.
Funding
This work was supported through an Australian Government Research Training Program Scholarship to J.D.B., a University of Western Australia Research Collaboration Award and an Australian Research Council Discovery Project to L.W.S.
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: J. Murray. [Google Scholar]
- 2.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: The Clarendon press. [Google Scholar]
- 3.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Zuk M, Kolluru GR. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438. ( 10.1086/420412) [DOI] [Google Scholar]
- 5.Robinson MR, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB. 2006. Live fast, die young: trade-offs between fitness components and sexually antagonistic selection on weaponry in Soay sheep. Evolution 60, 2168–2181. ( 10.1111/j.0014-3820.2006.tb01854.x) [DOI] [PubMed] [Google Scholar]
- 6.Brooks R. 2000. Negative genetic correlation between male sexual attractiveness and survival. Nature 406, 67–70. ( 10.1038/35017552) [DOI] [PubMed] [Google Scholar]
- 7.Zahavi A. 1975. Mate selection: a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 8.Grafen A. 1990. Sexual selection unhandicapped by the Fisher process. J. Theor. Biol. 144, 473–516. ( 10.1016/s0022-5193(05)80087-6) [DOI] [PubMed] [Google Scholar]
- 9.Roff DA. 2002. Life history evolution. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 10.Johnston SE, Gratten J, Berenos C, Pilkington JG, Clutton-Brock TH, Pemberton JM, Slate J. 2013. Life history trade-offs at a single locus maintain sexually selected genetic variation. Nature 502, 93–95. ( 10.1038/nature12489) [DOI] [PubMed] [Google Scholar]
- 11.Kotiaho JS. 2001. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol. Rev. 76, 365–376. ( 10.1017/s1464793101005711) [DOI] [PubMed] [Google Scholar]
- 12.Ingleby FC, Hosken DJ, Flowers K, Hawkes MF, Lane SM, Rapkin J, House CM, Sharma MD, Hunt J. 2014. Environmental heterogeneity, multivariate sexual selection and genetic constraints on cuticular hydrocarbons in Drosophila simulans. J. Evol. Biol. 27, 700–713. ( 10.1111/jeb.12338) [DOI] [PubMed] [Google Scholar]
- 13.Steiger S, Capodeanu-Nagler A, Gershman SN, Weddle CB, Rapkin J, Sakaluk SK, Hunt J. 2015. Female choice for male cuticular hydrocarbon profile in decorated crickets is not based on similarity to their own profile. J. Evol. Biol. 28, 2175–2186. ( 10.1111/jeb.12740) [DOI] [PubMed] [Google Scholar]
- 14.Lane SM, Dickinson AW, Tregenza T, House CM. 2016. Sexual Selection on male cuticular hydrocarbons via male–male competition and female choice. J. Evol. Biol. 29, 1346–1355. ( 10.1111/jeb.12875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas ML, Simmons LW. 2009. Sexual selection on cuticular hydrocarbons in the Australian field cricket, Teleogryllus oceanicus. BMC Evol. Biol. 9, 12 ( 10.1186/1471-2148-9-162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chenoweth SF, Blows MW. 2003. Signal trait sexual dimorphism and mutual sexual selection in Drosophila serrata. Evolution 57, 2326–2334. ( 10.1111/j.0014-3820.2003.tb00244.x) [DOI] [PubMed] [Google Scholar]
- 17.Van Homrigh A, Higgie M, McGuigan K, Blows MW. 2007. The depletion of genetic variance by sexual selection. Curr. Biol. 17, 528–532. ( 10.1016/j.cub.2007.01.055) [DOI] [PubMed] [Google Scholar]
- 18.Curtis S, Sztepanacz JL, White BE, Dyer KA, Rundle HD, Mayer P. 2013. Epicuticular compounds of Drosophila subquinaria and D. recens: identification, quantification, and their role in female mate choice. J. Chem. Ecol. 39, 579–590. ( 10.1007/s10886-013-0284-1) [DOI] [PubMed] [Google Scholar]
- 19.Steiger S, Ower GD, Stökl J, Mitchell C, Hunt J, Sakaluk SK. 2013. Sexual selection on cuticular hydrocarbons of male sagebrush crickets in the wild. Proc. R. Soc. B 280, 20132353 ( 10.1098/rspb.2013.2353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berson JD, Simmons LW. 2018. Sexual selection across sensory modalities: female choice of male behavioral and gustatory displays. Behav. Ecol. 29, 1096–1104. ( 10.1093/beheco/ary085) [DOI] [Google Scholar]
- 21.Gibbs AG, Rajpurohit S. 2010. Cuticular lipids and water balance. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds Blomquist GJ, Bagnéres A-G), pp. 100–120. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Blomquist GJ, Bagnères A-G. 2010. Introduction: history and overview of insect hydrocarbons. In Insect hydrocarbons: biology, biochemistry, and chemical ecology (eds GJ Blomquist, A-G Bagnéres), pp. 3–18. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Gibbs A, Pomonis JG. 1995. Physical properties of insect cuticular hydrocarbons: the effects of chain length, methyl-branching and unsaturation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 112, 243–249. ( 10.1016/0305-0491(95)00081-x) [DOI] [Google Scholar]
- 24.Gibbs AG. 2002. Lipid melting and cuticular permeability: new insights into an old problem. J. Insect. Physiol. 48, 391–400. ( 10.1016/S0022-1910(02)00059-8) [DOI] [PubMed] [Google Scholar]
- 25.Montooth KL, Gibbs AG. 2003. Cuticular pheromones and water balance in the house fly, Musca domestica. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 135, 457–465. ( 10.1016/S1095-6433(03)00115-6) [DOI] [PubMed] [Google Scholar]
- 26.Menzel F, Blaimer BB, Schmitt T. 2017. How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. Proc. R. Soc. B 284, 20161727 ( 10.1098/rspb.2016.1727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiger S, Stökl J. 2014. The role of sexual selection in the evolution of chemical signals in insects. Insects 5, 423–438. ( 10.3390/insects5020423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma MD, Hunt J, Hosken DJ. 2012. Antagonistic responses to natural and sexual selection and the sex-specific evolution of cuticular hydrocarbons in Drosophila simulans. Evolution 66, 665–677. ( 10.1111/j.1558-5646.2011.01468.x) [DOI] [PubMed] [Google Scholar]
- 29.Chung H, Carroll SB. 2015. Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. Bioessays 37, 822–830. ( 10.1002/bies.201500014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung H, Loehlin DW, Dufour HD, Vaccarro K, Millar JG, Carroll SB. 2014. A single gene affects both ecological divergence and mate choice in Drosophila. Science 343, 1148–1151. ( 10.1126/science.1249998) [DOI] [PubMed] [Google Scholar]
- 31.Foley BR, Telonis-Scott M. 2011. Quantitative genetic analysis suggests causal association between cuticular hydrocarbon composition and desiccation survival in Drosophila melanogaster. Heredity 106, 68–77. ( 10.1038/hdy.2010.40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hine E, McGuigan K, Blows MW. 2011. Natural selection stops the evolution of male attractiveness. Proc. Natl Acad. Sci. USA 108, 3659–3664. ( 10.1073/pnas.1011876108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosden TP, Reddiex AJ, Chenoweth SF. 2018. Artificial selection reveals sex differences in the genetic basis of sexual attractiveness. Proc. Natl Acad. Sci. USA 115, 5498–5503. ( 10.1073/pnas.1720368115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balakrishnan R, Pollack G. 1997. The role of antennal sensory cues in female responses to courting males in the cricket Teleogryllus oceanicus. J. Exp. Biol. 200, 511–522. [DOI] [PubMed] [Google Scholar]
- 35.Simmons LW, Thomas ML, Simmons FW, Zuk M. 2013. Female preferences for acoustic and olfactory signals during courtship: male crickets send multiple messages. Behav. Ecol. 24, 1099–1107. ( 10.1093/beheco/art036) [DOI] [Google Scholar]
- 36.Thomas ML, Simmons LW. 2008. Cuticular hydrocarbons are heritable in the cricket Teleogryllus oceanicus. J. Evol. Biol. 21, 801–806. ( 10.1111/j.1420-9101.2008.01514.x) [DOI] [PubMed] [Google Scholar]
- 37.Zuk M, Rotenberry JT, Tinghitella RM. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2, 521–524. ( 10.1098/rsbl.2006.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tinghitella RM. 2008. Rapid evolutionary change in a sexual signal: genetic control of the mutation ‘flatwing’ that renders male field crickets (Teleogryllus oceanicus) mute. Heredity 100, 261–267. ( 10.1038/sj.hdy.6801069) [DOI] [PubMed] [Google Scholar]
- 39.Pascoal S, Cezard T, Eik-Nes A, Gharbi K, Majewska J, Payne E, Ritchie MG, Zuk M, Bailey NW. 2014. Rapid convergent evolution in wild crickets. Curr. Biol. 24, 1369–1374. ( 10.1016/j.cub.2014.04.053) [DOI] [PubMed] [Google Scholar]
- 40.Simmons LW, Thomas ML, Gray B, Zuk M. 2014. Replicated evolutionary divergence in the cuticular hydrocarbon profile of male crickets associated with the loss of song in the Hawaiian archipelago. J. Evol. Biol. 27, 2249–2257. ( 10.1111/jeb.12478) [DOI] [PubMed] [Google Scholar]
- 41.Lynch M., Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 42.Kruuk LEB. 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. B 359, 873–890. ( 10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson AJ, Reale D, Clements MN, Morrissey MM, Postma E, Walling CA, Kruuk LEB, Nussey DH. 2010. An ecologist's guide to the animal model. J. Anim. Ecol. 79, 13–26. ( 10.1111/j.1365-2656.2009.01639.x) [DOI] [PubMed] [Google Scholar]
- 44.Charmantier A., Garant D., Kruuk L.E.B. 2014. Quantitative genetics in the wild. Oxford, UK: Oxford University Press. [Google Scholar]
- 45.van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 46.Houle D. 1991. Genetic covariance of fitness correlates: what genetic correlations are made of and why it matters. Evolution 45, 630–648. ( 10.2307/2409916) [DOI] [PubMed] [Google Scholar]
- 47.Simmons LW. 2012. Resource allocation trade-off between sperm quality and immunity in the field cricket, Teleogryllus oceanicus. Behav. Ecol. 23, 168–173. ( 10.1093/beheco/arr170) [DOI] [Google Scholar]
- 48.Bateman PW, Gilson LN, Ferguson JWH. 2001. Male size and sequential mate preference in the cricket Gryllus bimaculatus. Anim. Behav. 61, 631–637. ( 10.1006/anbe.2000.1617) [DOI] [Google Scholar]
- 49.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 50.Ingleby F. 2015. Insect cuticular hydrocarbons as dynamic traits in sexual communication. Insects 6, 732–742. ( 10.3390/insects6030732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otte T, Hilker M, Geiselhardt S. 2018. Phenotypic plasticity of cuticular hydrocarbon profiles in insects. J. Chem. Ecol. 44, 235–247. ( 10.1007/s10886-018-0934-4) [DOI] [PubMed] [Google Scholar]
- 52.Thomas ML, Simmons LW. 2011. Short-term phenotypic plasticity in long-chain cuticular hydrocarbons. Proc. R. Soc. B 278, 3123–3128. ( 10.1098/rspb.2011.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas ML, Gray B, Simmons LW. 2011. Male crickets alter the relative expression of cuticular hydrocarbons when exposed to different acoustic environments. Anim. Behav. 82, 49–53. ( 10.1016/j.anbehav.2011.03.023) [DOI] [Google Scholar]
- 54.Pascoal S, Mendrok M, Mitchell C, Wilson AJ, Hunt J, Bailey NW. 2016. Sexual selection and population divergence I: the influence of socially flexible cuticular hydrocarbon expression in male field crickets (Teleogryllus oceanicus). Evolution 70, 82–97. ( 10.1111/evo.12839) [DOI] [PubMed] [Google Scholar]
- 55.McGuigan K, Van Homrigh A, Blows MW. 2008. An evolutionary limit to male mating success. Evolution 62, 1528–1537. ( 10.1111/j.1558-5646.2008.00379.x) [DOI] [PubMed] [Google Scholar]
- 56.Delcourt M, Blows MW, Aguirre JD, Rundle HD. 2012. Evolutionary optimum for male sexual traits characterized using the multivariate Robertson-price identity. Proc. Natl Acad. Sci. USA 109, 10 414–10 419. ( 10.1073/pnas.1116828109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gershman SN, Rundle HD. 2016. Level up: the expression of male sexually selected cuticular hydrocarbons is mediated by sexual experience. Anim. Behav. 112, 169–177. ( 10.1016/j.anbehav.2015.11.025) [DOI] [Google Scholar]
- 58.Kruuk LEB, Hadfield JD. 2007. How to separate genetic and environmental causes of similarity between relatives. J. Evol. Biol. 20, 1890–1903. ( 10.1111/j.1420-9101.2007.01377.x) [DOI] [PubMed] [Google Scholar]
- 59.Roff DA, Wilson AJ. 2014. Quantifying genotype-by-environment interactions in laboratory systems. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 60.Morrissey MB, Ruxton GD. 2018. Multiple regression is not multiple regressions: the meaning of multiple regression and the non-problem of collinearity. Phil. Theor. Pract. Biol. 10, 3. [Google Scholar]
- 61.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 62.Butler DG, Cullis BR, Gilmour AR, Gogel BJ. 2009. Mixed models for S language environments: ASReml-R reference manual Brisbane, Australia: Queensland Government. [Google Scholar]
- 63.Diez DM. 2013. OIsurv: Survival analysis supplement to OpenIntro guide. R package version 0.2.
- 64.Wickham H. 2009. Ggplot2: elegant graphics for data analysis. New York, NY: Springer-Verlag. [Google Scholar]
- 65.Canty A., Ripley B. 2016. Bootstrap R (S-Plus) Functions. R package version 1.3-18.
- 66.Walling CA, Morrissey MB, Foerster K, Clutton-Brock TH, Pemberton JM, Kruuk LEB. 2014. A multivariate analysis of genetic constraints to life history evolution in a wild population of red deer. Genetics 198, 1735–1749. ( 10.1534/genetics.114.164319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roff DA. 2001. The threshold model as a general purpose normalizing transformation. Heredity 86, 404 ( 10.1046/j.1365-2540.2001.00844.x) [DOI] [PubMed] [Google Scholar]
- 68.Olausson A, Rönningen K. 1975. Estimation of genetic parameters for threshold characters. Acta Agric. Scand. 25, 201–208. ( 10.1080/00015127509435039) [DOI] [Google Scholar]
- 69.Buzatto BA, Kotiaho JS, Tomkins JL, Simmons LW. 2015. Intralocus tactical conflict: genetic correlations between fighters and sneakers of the dung beetle Onthophagus taurus. J. Evol. Biol. 28, 730–738. ( 10.1111/jeb.12598) [DOI] [PubMed] [Google Scholar]
- 70.King KJ, Sinclair BJ. 2015. Water loss in tree weta (Hemideina): adaptation to the montane environment and a test of the melanisation-desiccation resistance hypothesis. J. Exp. Biol. 218, 1995–2004. ( 10.1242/jeb.118711) [DOI] [PubMed] [Google Scholar]
- 71.Ramniwas S, Kajla B, Dev K, Parkash R. 2012. Direct and correlated responses to laboratory selection for body melanisation in Drosophila melanogaster: support for melanism-desiccation resistance hypothesis. J. Exp. Biol. 216(Pt 7), 1244–1254. ( 10.1242/jeb.076166) [DOI] [PubMed] [Google Scholar]
- 72.Gibbs AG, Chippindale AK, Rose MR. 1997. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 200, 1821–1832. [DOI] [PubMed] [Google Scholar]
- 73.Gefen E, Marlon AJ, Gibbs AG. 2006. Selection for desiccation resistance in adult Drosophila melanogaster affects larval development and metabolite accumulation. J. Exp. Biol. 209, 3293–3300. ( 10.1242/jeb.02397) [DOI] [PubMed] [Google Scholar]
- 74.Chippindale AK, Gibbs AG, Sheik M, Yee KJ, Djawdan M, Bradley TJ, Rose MR. 1998. Resource acquisition and the evolution of stress resistance in Drosophila melanogaster. Evolution 52, 1342–1352. ( 10.2307/2411304) [DOI] [PubMed] [Google Scholar]
- 75.Gibbs AG, Louie AK, Ayala JA. 1998. Effects of temperature on cuticular lipids and water balance in a desert Drosophila: is thermal acclimation beneficial? J. Exp. Biol. 201, 71–80. [DOI] [PubMed] [Google Scholar]
- 76.Wang Q, Goodger JQD, Woodrow IE, Elgar MA. 2016. Location-specific cuticular hydrocarbon signals in a social insect. Proc. R. Soc. B 283, 20160310 ( 10.1098/rspb.2016.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gibbs A, Crowe JH. 1991. Intra-individual variation in cuticular lipids studied using Fourier transform infrared spectroscopy. J. Insect. Physiol. 37, 743–748. ( 10.1016/0022-1910(91)90108-C) [DOI] [Google Scholar]
- 78.Mitchell-Olds T, Shaw RG. 1987. Regression analysis of natural selection: statistical inference and biological interpretation. Evolution 41, 1149–1161. ( 10.1111/j.1558-5646.1987.tb02457.x) [DOI] [PubMed] [Google Scholar]
- 79.Lockey KH. 1988. Lipids of the insect cuticle: origin, composition and function. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 89, 595–645. ( 10.1016/0305-0491(88)90305-7) [DOI] [Google Scholar]
- 80.Berson JD, Zuk M, Simmons LW. 2019. Data from: Natural and sexual selection on cuticular hydrocarbons: a quantitative genetic analysis Dryad Digital Repository. ( 10.5061/dryad.r2j05j8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Berson JD, Zuk M, Simmons LW. 2019. Data from: Natural and sexual selection on cuticular hydrocarbons: a quantitative genetic analysis Dryad Digital Repository. ( 10.5061/dryad.r2j05j8) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.r2j05j8 [80].