Abstract
Background
Shigella dysentery is a relatively common illness and occasionally causes death, worldwide. Mild symptoms are self‐limiting but in more severe cases, antibiotics are recommended for cure and preventing relapse. The antibiotics recommended are diverse, have regional differences in sensitivity, and have side effects.
Objectives
To evaluate the efficacy and safety of antibiotics for treating Shigella dysentery.
Search methods
In June 2009 we identified all relevant trials from the following databases: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, issue 4), MEDLINE, EMBASE, LILACS and the metaRegister of Controlled Trials (mRCT). We also checked conference proceedings for relevant abstracts, and contacted researchers, organizations, and pharmaceutical companies.
Selection criteria
Randomized controlled trials of antibiotics for Shigella dysentery.
Data collection and analysis
Four authors, working in pairs, independently assessed trial eligibility, methodological quality, and extracted data. We calculated risk ratios (RR) with 95% confidence intervals (CI) for dichotomous data, and used the random‐effects model for significant heterogeneity. We explored possible sources of heterogeneity, when present, in subgroup analyses of participant age and percentage of participants with confirmed Shigella infection.
Main results
Sixteen trials (1748 participants), spanning four decades and with differing sensitivity to Shigella isolates, met the inclusion criteria. Seven were judged to be at risk of bias due to inadequate allocation concealment or blinding, and 12 due to incomplete reporting of outcome data. Limited data from one three‐armed trial of people with moderately severe illness suggest that antibiotics reduce the episodes of diarrhoea at follow‐up (furazolidone versus no drug RR 0.21, 95% CI 0.09 to 0.48, 73 participants; cotrimoxazole versus no drug RR 0.30, 95% CI 0.15 to 0.59, 76 participants).
There was insufficient evidence to consider any class of antibiotic superior in efficacy in treating Shigella dysentery, but heterogeneity for some comparisons limits confidence in the results. All the antibiotics studied were safe. There was inadequate evidence regarding the role of antibiotics in preventing relapses.
Authors' conclusions
Antibiotics reduce the duration of Shigella dysentery.
Regularly updated local or regional antibiotic sensitivity patterns to different species and strains of Shigella are required to guide empiric therapy. More trials adhering to standard guidelines are required to evaluate the role of antibiotics in the treatment of severe forms of Shigella dysentery and in groups who are at high risk of complications.
15 April 2019
No update planned
Other
There has been a review of this topic in Lancet Infectious Diseases: Tickell 2017 https://doi.org/10.1016/S2214‐109X(17)30392‐3. Thus, this is not a current priority for the CIDG to update.
Plain language summary
Antibiotic therapy for Shigella dysentery
Shigellosis is a bacterial infection of the colon that can cause diarrhoea, dysentery (diarrhoea with blood and/or mucus) and may lead to death. It occurs mainly in low‐ and middle‐income countries where overcrowding and poor sanitation exist, and may lead to around 1.1 million deaths per year globally, mostly in children under five years.
The intention of giving antibiotics in shigellosis is to speed recovery, reduce the seriousness of the disease, and reduce the length of time patients are infective. However, some antibiotics can have serious side effects while others may not be effective against the Shigella bacteria.
The review examined both the effectiveness and the safety of antibiotics in treating Shigella dysentery. While antibiotics tested here appeared safe and effective, there was insufficient evidence to suggest which antibiotics were superior. More well designed trials will help inform decision making.
Summary of findings
Background
Description of the condition
Shigellosis is a bacterial infection of the colon that causes diarrhoea and can lead to death. Dysentery (frequent mucoid or bloody stools) when caused by Shigella is called Shigella dysentery. Of the estimated 164.7 million Shigella diarrhoeal episodes occurring globally every year, most occur in developing countries (99%) and mainly in children (69%) (WHO 2006). Of the 1.1 million deaths due to Shigella, 69% are in children aged less than five years (Kotloff 1999; WHO 2006).
Microbiology and mode of spread
Shigella dysenteriae, S. flexneri, S. sonnei, and S. boydii are the four species of small, Gram‐negative, non‐motile bacilli that cause shigellosis, and all but S. sonnei have more than one genetically distinct subtype (serotype) (von Seidlein 2006). The species distribution varies globally; for example, S. flexneri was reported to be most prevalent in India (58%, Dutta 2002) and Rwanda (68%, Bogaerts 1983), while S. sonnei was the most frequently detected species in Thailand (85%, von Seidlein 2006), Israel (48.8%, Mates 2000), and the USA (75%, Gupta 2004; Shiferaw 2004).
Shigellae are transmitted by the faeco‐oral route, via direct person‐to‐person contact, and via food, water, and inanimate objects. Only a small number of ingested bacteria are required to produce illness. The disease is communicable as long as an infected person excretes the organism in the stool, which can extend up to four weeks from the onset of illness. Secondary attack rates, the number of exposed persons developing the disease within one to four days following exposure to the primary case (Park 2005), can be as high as 40% among household contacts (Sur 2004).
Shigellosis occurs predominantly in developing countries and is most common where overcrowding and poor sanitation exist. It occurs in densely populated areas and institutions where populations are in close contact with each other, such as day‐care centres, cruise ships, institutions for people with mental or psychological problems, and military barracks (Shane 2003; Gupta 2004).
Clinical features
The clinical manifestation of shigellosis ranges from an asymptomatic illness to bacteraemia and sepsis. Symptoms include fever, diarrhoea and/or dysentery with abdominal cramps and ineffectual and painful straining at stool or in urinating (Niyogi 2005). Shigellosis may be associated with mild to life‐threatening complications, such as rectal prolapse, arthralgia (painful joints), arthritis, intestinal perforation, and toxic mega colon (extreme inflammation and distension of the colon), central nervous disorders, convulsions, enteropathy (protein‐losing disease of the intestines), electrolyte imbalance of salts, and sepsis (Sur 2004; WHO 2005b). About 3% of those infected with S. flexneri and who are genetically predisposed can develop Reiter's syndrome (pains in their joints, irritation of the eyes, and painful urination) that can lead to a difficult to treat chronic arthritis (CDC 2005). Haemolytic uraemic syndrome (a complication resulting in kidney failure, bleeding, and anaemia) and leukemoid reaction (blood findings resembling leukaemia) complicate infection due to S. dysenteriae type 1 and may be fatal (Sinha 1987). S. dysenteriae type 1 is the only Shigella species with chromosomal genes encoding the protein known as Shiga toxin (Thorpe 2001).
Diagnosis
The clinical features of fever with blood and/or mucous diarrhoea associated with abdominal pain suggest that the aetiology of diarrhoea is Shigella. Routine microscopy of fresh stool is a simple screening test that is cheap, rapid, and easy to perform; and visualization of numerous poly‐morphonucleocytes suggests a bacterial aetiology. Definite diagnosis of shigellosis can only be made by stool culture (WHO 2005a). However, Shigella species die rapidly in unfavourable environments and stool culture should ideally be supplemented by attempts to identify Shigella DNA using polymerase chain reaction (PCR) (von Seidlein 2006).
Relapse
Clinical relapse can occur. This manifests as an initial clinical improvement or apparent cure with the treatment, followed by the recurrence of diarrhoea after the course of drug treatment is completed. In some instances people have sought the continued presence of Shigella in cultures of stool after the treatment, irrespective of apparent clinical recovery and have documented these as bacteriological failures (Martin 2000), indicative of the potential for relapse. Relapse is an important indicator of treatment failure, though it is clinically difficult to differentiate a relapse of infection with the same species or serotype of Shigella without additional testing for Shigella DNA using PCR analysis (von Seidlein 2006).
Mortality
The case‐fatality rate is estimated to be less than 1% among those with mild illness (WHO 2005a), which is usually self‐limiting (CDC 2005), and those affected are usually treated as out‐patients. However, case fatality is as high as 15% among patients with S. dysenteriae type 1 who require hospitalization; this rate is increased by delayed arrival and treatment with ineffective antibiotics. Infants, non‐breast fed children, children recovering from measles, malnourished children, and adults older than 50 years have a more severe illness and a greater risk of death (WHO 2005a).
Shigella and HIV infection
Human immunodeficiency virus (HIV) infection may be an important risk factor for Shigella infection. Particularly in HIV‐positive people, shigellosis is associated with extensive illness, including Shigella septicaemia, and increased health‐care expenditures. The diagnosis of shigellosis in an otherwise healthy adult without obvious exposure risk for Shigella should prompt consideration of the possibility of HIV infection (Huebner 1993; Baer 1999).
Description of the intervention
The World Health Organization (WHO) recommends that all suspected cases of shigellosis based on clinical features be treated with effective antimicrobials (antibiotics). The choice of antimicrobial drug has changed over the years as resistance to antibiotics has occurred, with different patterns of resistance being reported around the world. The following antibiotics were used to treat Shigella dysentery:
class: beta‐lactams: ampicillin, amoxicillin, first and second generation cephalosporins (cefixime, ceftriaxone) and pivmecillinam;
class: quinolones: nalidixic acid, ciprofloxacin, norfloxacin, ofloxacin;
class: macrolides: azithromycin; others: sulphonamides, tetracycline, cotrimoxazole, and furazolidone.
The WHO now recommends that clinically diagnosed cases of Shigella dysentery be treated with ciprofloxacin as first line treatment, and pivmecillinam, ceftriaxone, or azithromycin as second line treatment and lists the others as ineffective (WHO 2005a). However, resistance to quinolones has also been observed since the late 1990s, and some authors have questioned the effectiveness of this class for Shigella (Datta 2003; Sarkar 2003; Sur 2003; Pazhani 2004; Talukder 2004).
Why it is important to do this review
When an effective antibiotic is given, clinical improvement is anticipated within 48 hours (WHO 2005a). This lessens the risk of serious complications and death, shortens the duration of symptoms, and hastens the elimination of Shigella and the subsequent spread of infection (WHO 2005a). Since the antibiotics used for treating shigellosis can have adverse effects (Table 9; BNF 2007), some life‐threatening, the clinician is faced with a dilemma in choosing an appropriate drug to treat shigellosis. This drug must be effective, locally available at affordable costs, be associated with minimum adverse effects and be sensitive to local Shigella species and strains. We undertook this review in the hope of identifying such a drug or group of drugs.
1. Known adverse effects of antibiotics used to treat Shigella dysentery^.
| Antibiotic | Life threatening | Discontinuation^^ | Other |
| Tetracycline | Anaphylaxis | Oesophageal irritation, antibiotic‐associated colitis, headache and visual disturbances | In children under 12 years of age causes dental hypoplasia and staining, benign intracranial hypertension |
| Chloramphenicol | Blood disorders, peripheral and optic neuritis, erythema multiforme | Dyspepsia | — |
| Ampicillin | Hypersensitivity reactions | Diarrhoea | — |
| Co‐trimoxazole or trimethoprim ‐ sulphamethoxazole | Stevens‐Johnson syndrome | Diarrhoea, rash | — |
| Fluoroquinolones | Hypersensitivity | Dyspepsia, headache, hypotension | Pruritis, tachycardia |
| Norfloxacin | — | Dyspepsia, headache, hypotension | Euphoria, tinnitus, polyneuropathy |
| Ciprofloxacin | — | Dyspepsia, headache, hypotension | Hot flushes, sweating, tenosynovitis |
| Ofloxacin | — | Dyspepsia, headache, hypotension | Anxiety, unsteady gait |
| Azithromycin | Hypersensitivity | Dyspepsia, flatulence, headache | — |
| Ceftriaxone | Hypersensitivity reactions | Diarrhoea, headache, abdominal discomfort | — |
| Nalidixic acid | — | Same as in fluoroquinolones | Toxic psychosis, increased intracranial tension, cranial nerve palsy |
| Rifaximin | Allergic reactions | Allergic reactions | — |
| Cefixime | Hypersensitivity reactions | Flatulence, headache, abdominal pain, defecation urgency, nausea, constipation, pyrexia, vomiting | — |
| Pivmecillinam | — | Same as ampicillin, dyspepsia | — |
^Source: BNF 2007. ^^Can result in discontinuation of treatment.
Objectives
To evaluate the efficacy and safety of antibiotics for treating Shigella dysentery.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Adults and children with clinical symptoms suggestive of Shigella dysentery. Both hospitalized and non‐hospitalized participants were included.
Types of interventions
Intervention
Antibiotics, irrespective of the dose or route of administration.
Control
Other antibiotic of a different class (irrespective of the dose or route of administration), placebo, or no drug.
We included trials that used additional interventions if the interventions were used in all treatment arms.
Types of outcome measures
Primary outcomes
Diarrhoea at follow up.
Relapse, defined as the reappearance of diarrhoea associated with Shigella in the stool or dysentery during follow up.
Secondary outcomes
Fever at follow up: defined as body temperature above 37.0 ºC or 98.6 ºF.
Time to cessation of fever.
Time to cessation of diarrhoea.
Time to cessation of blood in stools.
Total number of stools per day.
Bacteriological cure: defined as a negative stool culture at the end of a specified time period after treatment.
Duration of hospital stay.
Development of severe complications.
Death.
Serious adverse events (i.e. those that are life‐threatening or require hospitalization); those that lead to discontinuation of treatment; other types of adverse events.
Search methods for identification of studies
We identified all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases using the strategies and search terms set out in Table 10: the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, issue 4); MEDLINE (1966 to June 2009); EMBASE (1974 to June 2009); and LILACS (1982 to June 2009). We also searched the metaRegister of Controlled Trials (mRCT) using 'shigell*' as the search term (June 2009).
2. Detailed search strategies.
| Search set | CIDG SR^ | CENTRAL | MEDLINE^^ | EMBASE^^ | LILACS^^ |
| 1 | Shigell* | Shigell* | Shigell* | Shigell$ | Shigell* |
| 2 | Dysentery | DYSENTERY, BACILLARY | DYSENTERY, BACILLARY | SHIGELLOSIS | Dysentery |
| 3 | 1 or 2 | 1 or 2 | 1 or 2 | DYSENTERY | 1 or 2 |
| 4 | antibiotic* | ANTI‐BACTERIAL AGENTS/THERAPEUTIC USE | ANTI‐BACTERIAL AGENTS/THERAPEUTIC USE | 1 or 2 or 3 | antibiotic* |
| 5 | tetracycline* | ANTI‐INFECTIVE AGENTS/THERAPEUTIC USE | ANTI‐INFECTIVE AGENTS/THERAPEUTIC USE | tetracycline$ | tetracycline* |
| 6 | chloramphenicol | antibiotic* | antibiotic* | chloramphenicol | chloramphenicol |
| 7 | ampicillin* | tetracycline* | tetracycline* | ampicillin | ampicillin |
| 8 | co‐trimoxazole | chloramphenicol | chloramphenicol | co‐trimoxazole | co‐trimoxazole |
| 9 | fluoroquinolone* | ampicillin | ampicillin | fluoroquinolone$ | fluoroquinolone* |
| 10 | quinolone* | co‐trimoxazole | co‐trimoxazole | quinolone$ | quinolone* |
| 11 | norfloxacin | fluoroquinolone* | fluoroquinolone* | norfloxacin | norfloxacin |
| 12 | ciprofloxacin | quinolone* | quinolone* | ciprofloxacin | ciprofloxacin |
| 13 | ofloxacin | norfloxacin | norfloxacin | ofloxacin | ofloxacin |
| 14 | azithromycin | ciprofloxacin | ciprofloxacin | azithromycin | azithromycin |
| 15 | ceftriaxone | ofloxacin | ofloxacin | ceftriaxone | ceftriaxone |
| 16 | nalidixic acid | azithromycin | azithromycin | nalidixic acid | nalidixic acid |
| 17 | pivmecillinam | ceftriaxone | ceftriaxone | rifaximin | rifaximin |
| 18 | 4‐17/or | nalidixic acid | nalidixic acid | cefixime | cefixime |
| 19 | 3 and 18 | rifaximin | rifaximin | trimethoprim‐sulfamethoxazole | trimethoprim‐sulfamethoxazole |
| 20 | — | cefixime | cefixime | antibiotic$ | pivmecillinam |
| 21 | — | trimethoprim‐sulfamethoxazole | trimethoprim‐sulfamethoxazole | pivmecillinam | 4‐20/or |
| 22 | — | pivmecillinam | pivmecillinam | 5‐21/or | 3 and 21 |
| 23 | — | 4‐22/or | 4‐22/or | Limit 22 to human | — |
| 24 | — | 3 and 23 | 3 and 23 | — | — |
| 25 | — | — | Limit 24 to human | — | — |
^Cochrane Infectious Diseases Group Specialized Register. ^^Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2006); upper case: MeSH or EMTREE heading; lower case: free text term.
Searching other resources
In Table 11 we list the conference proceedings searched for relevant abstracts, individual researchers working in this field contacted, organizations and pharmaceutical companies contacted to identify unpublished and ongoing trials, along with the dates when this was done. We also checked the reference lists of all studies identified by the above methods.
3. Search strategy: proceedings, organizations, and pharmaceutical companies.
| Type | Detail |
| Conference proceeding | ‐ Commonwealth Congress on Diarrhoea and Malnutrition: 8th, Dhaka, Bangladesh, 6 to 8 February 2006 (searched on 12 April 2007) ‐ Asian Conference on Diarrhoeal Diseases and Nutrition: 10th, Dhaka, Bangladesh, 7 to 9 December 2003 (searched on 13 April 2007) ‐ Annual Scientific Conference: 10th Dhaka, Bangladesh, 11 to 13 June 2002 (searched on 13 April 2007) ‐ Annual Meeting of Infectious Disease Society of America: 44th, Toronto, Ontario, Canada, 12 to 15 October 2006; 43rd, San Francisco, California, 6 to 9 October 2005; 42nd, Boston, Massachusetts, USA, 30 September to 3 October 2004 (searched on 18 March 2008) ‐ Interscience Conference on Antimicrobial Agents and Chemotherapy: 46th, San Francisco, California, 27 to 30 September 2006; 45th, Washington DC, USA, 16 to 19 December 2005; 44th, Washington DC, USA, 30 October to 2 November, 2004 (searched on 18 March 2008) ‐ European Congress of Clinical Microbiology and Infectious Diseases: 16th, Nice, France, 1 to 4 April 2006; 15th, 2 to 5 April 2005 (searched on 18 March 2008) ‐ International Congress on Infectious Diseases: 12th, Lisbon, Portugal, 15 to 18 June 2006; 11th, Cancun, Mexico, 4 to 7 March 2004 (searched on 18 March 2008) ‐ Annual Meeting of The European Society for Paediatric Infectious Disease: 24th, Basel, Switzerland, 3 to 5 May 2006 (searched on 18 March 2008) ‐ Western Pacific Congress of Chemotherapy and Infectious Diseases: 10th, Fukuoka, Japan, 3 to 6 December 2006 (searched on 18 March 2008) ‐ European Congress of Chemotherapy and Infection: 8th, Budapest, Hungary, 25 to 28 October 2006 (searched on 18 March 2008) |
| Organizations | ‐ Liverpool School of Tropical Medicine (contacted on 11 April 2007) ‐ World Health Organization (contacted on 17 March 2008) ‐ American Society of Tropical Medicine and Hygiene (contacted on 15 April 2007) ‐ International Society of Tropical Pediatrics (contacted on 15 April 2007) ‐ South East Asian Ministers Education Organization (SEAMEO) TROPMED Network (contacted on 17 March 2008) ‐ International Center for Diarrhoeal Disease Research in Bangladesh (contacted on 21 April 2007) |
| Pharmaceutical companies | ‐ Goldshield Pharmaceuticals Ltd (tetracycline, Deteclo; chloramphenicol, Chloromycetin) ‐ contacted on 17 March 2008 ‐ Chemidex (ampicillin, Penbritin) ‐ contacted on 17 March 2008 ‐ GlaxoSmithKline (co‐trimoxazole, Septrin) ‐ contacted on 17 March 2008 ‐ Merck Sharp & Dohme Ltd (norfloxacin, Utinor) ‐ contacted on 17 March 2008 ‐ Bayer (ciprofloxacin, Ciproxin) ‐ contacted on 20 April 2007 ‐ Aventis Pharma (ofloxacin, Tarivid) ‐ contacted on 15 April 2007 ‐ Pfizer (azithromycin, Zithromax) ‐ contacted on 17 March 2008 ‐ Roche (ceftriaxone, Rocephin) ‐ contacted on 20 April 2007 ‐ Rosemont Pharmaceuticals Ltd (nalidixic acid, Uriben) ‐ contacted on 13 April 2007 ‐ Salix Pharmaceuticals (rifaximin, Xifaxan) ‐ contacted on 17 March 2008 ‐ Rhone‐Poulenc Rorer (cefixime, Suprax) ‐ contacted on 17 March 2008 ‐ LEO pharma (pivmecillinam, Selexid) ‐ contacted on 17 March 2008 |
Data collection and analysis
Selection of studies
Two pairs of authors (PC and KVD, and SMJ and VS ) independently assessed the results of the literature search to determine whether the title or abstract of each trial cited was an RCT . We retrieved the full reports of all trials considered by one or both pairs of authors as potentially relevant as well as those that were unclear from scrutinizing the abstracts. Each pair used a standard eligibility form based on the inclusion and exclusion criteria to assess the trials. We resolved disagreements through discussion. If eligibility was uncertain due to unclear or inadequate information, we attempted to contact the trial authors for clarification. The reasons for excluding studies were noted in the 'Characteristics of excluded studies' table. Each trial report was scrutinized to ensure that multiple publications from the same trial are included only once, and all reports were linked to the original trial report in the reference list of included studies.
Data extraction and management
The pairs of authors independently extracted data from the trials using pre‐tested data extraction forms. We extracted data on the inclusion and exclusion criteria for the participants, treatment/intervention given, total number randomized, number of participants in each group for all outcomes, drop‐outs, and withdrawals and numbers experiencing each outcome. For every outcome, we extracted the number analysed and the number randomized in each treatment group to allow for the assessment of losses to follow up. Any disagreements about data extracted were resolved by referring to the trial report and by discussion. Where data were insufficient or missing, attempts were made to contact the trial authors.
For continuous outcomes, we extracted the arithmetic mean values, standard deviations, and the number of participants in whom the outcome was assessed in each of the two groups. We noted whether the numbers assessed in the trial were the number of participants that completed the trial or the number randomized. If medians were reported we extracted ranges, or interquartile ranges.
Assessment of risk of bias in included studies
The pairs of authors independently assessed the risk of bias in each included trial for the following six components: sequence generation, allocation concealment, blinding or masking, incomplete outcome data, selective outcome reporting, and other sources of bias. For each of these components, we assigned a judgment regarding the risk of bias as 'yes', 'no', or 'unclear' (Higgins 2008). We recorded follow up to be adequate if more than 90% of the randomized participants were included in the final analysis, inadequate if less than or equal to 90%, or unclear if this information was not available from the report or trial authors. We recorded these assessments in the standard table in RevMan 5 (Review Manager 2008), and summarized them in 'Risk of bias' tables and a graph (Figure 1; Figure 2). We used these assessments to perform a sensitivity analysis based on methodological quality when appropriate. We attempted to contact the trial authors for clarification when methodological details were unclear. We resolved differences by discussion and by contacting an Editor with the Cochrane Infectious Diseases Review Group.
1.
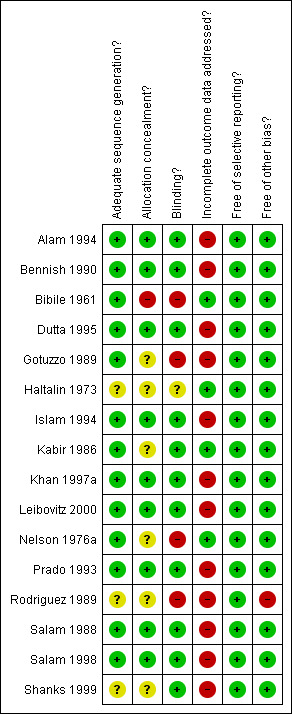
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
2.
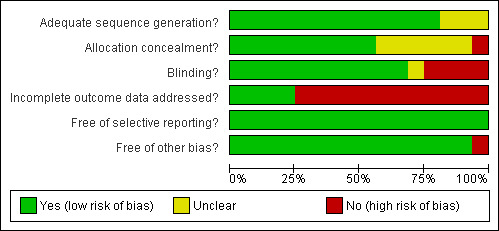
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
The measures of treatment effect used were risk ratio (RR) for dichotomous outcomes and mean difference for continuous outcomes with their 95% confidence intervals (CIs).
Dealing with missing data
Where possible, we extracted data to allow an intention‐to‐treat analysis in which all randomized participants were analysed in the groups to which they were originally assigned. If there was discrepancy in the number randomized and the numbers analysed in each treatment group, we calculated the percentage loss to follow up in each group and reported this information. For dichotomous outcomes, we recorded the number of participants experiencing the event and the number analysed in each treatment group. We assigned those lost to follow up the worse outcome, except for the outcome of death, since it would be unreasonable to assume that all those who were lost to follow up died.
Assessment of heterogeneity
We determined the presence of statistical heterogeneity among the same interventions by examining the forest plot and by performing the Chi2 test for heterogeneity using a P value of 0.10 to determine statistical significance. The I2 statistic was used to quantify inconsistency across trials and a value greater than 50% was considered as substantial heterogeneity (Deeks 2005).
Assessment of reporting biases
All studies were assessed for adequacy of reporting of data for pre‐stated outcomes and for selective reporting of outcomes. We noted judgements based on the risk of selective reporting in the 'Risk of bias' table for each study in the 'Characteristics of included studies' table.
Had there been sufficient trials we would have evaluated asymmetry in the funnel plot as an indication of publication bias.
Data synthesis
The first two authors entered data into Review Manager 2008 using double‐data entry. PC synthesized the data, which the co‐authors checked. All results are presented with 95% CIs. The main comparisons were between any antibiotic drug and placebo, and any antibiotic drug and another antibiotic drug of a different class.
We synthesized dichotomous data using pooled and weighted RRs. Continuous data summarized by arithmetic means and standard deviations were combined using the weighted mean differences.
We used the fixed‐effect model to synthesize data if heterogeneity was not substantial. When there was substantial heterogeneity and this could not be explained by subgroup analysis, we synthesized data using the random‐effects model and recommended a cautious interpretation of the pooled result.
Subgroup analysis and investigation of heterogeneity
When there was significant statistical heterogeneity, we explored the possible sources using the following subgroup analyses: participant age (adults versus children) and percentage of participants with confirmed Shigella infection.
Sensitivity analysis
We performed sensitivity analyses for primary outcomes to assess the robustness of the meta‐analysis among the same interventions by calculating the results using all trials and then excluding trials of a lower methodological quality (i.e. trials with inadequate generation of allocation sequence and allocation concealment, trials that were not double blind, and trials where less than or equal to 90% of randomized participants were analysed).
Results
Description of studies
Results of the search
Out of 265 studies retrieved by the search, we obtained full texts of 123 studies. The rest were excluded as they were neither RCTs nor studies of antibiotic therapy for Shigella. Of the 123 studies, 16 parallel group, individually randomized trials met inclusion criteria (see 'Characteristics of included studies') and are summarized below. The reasons for excluding the other 106 trials are recorded in the 'Characteristics of excluded studies' table. One study awaits assessment (Carbo 1981).
Included studies
Location, setting and length of follow up
Seven trials were conducted in Bangladesh, all at the International Centre for Diarrhoeal Disease Research (ICDDR,B). Two trials were from the United States of America (Haltalin 1973; Nelson 1976a) and one each from the following countries: India (Dutta 1995), Sri Lanka (Bibile 1961), Peru (Gotuzzo 1989), Israel (Leibovitz 2000), Guatemala (Prado 1993), Mexico (Rodriguez 1989), and Kenya (Shanks 1999). Twelve trials were carried out in hospitalized patients, three in out‐patients and one did not mention the setting. The trials used different lengths of follow up: eight trials were for six days, three trials for five days, two trials for 14 days and one trial each for seven days, 10 days, and six months.
Participants
The trials included a total of 1748 participants. All trials but one (Haltalin 1973) were randomized based on clinical symptoms of dysentery and prior to bacteriological confirmation. People with neither blood nor mucus in stools were excluded. Haltalin 1973 randomized participants after a presumptive confirmation of Shigella by immunofluorescence study of rectal swabs. Dutta 1995 did not seek microbiological confirmation for Shigella by culture of stool samples or rectal swabs. In the remaining trials, only the data from participants with microbiologically confirmed Shigella were reported and thus only those data were included in the analyses. Ten trials were carried out only in children, five in adults, and one included both. Among the 10 trials in children, only one (Dutta 1995) included malnourished children (11 of 72) but did not provide data on them separately. Two trials excluded children with malnutrition and the remaining seven trials did not provide such information. None of the trials reported the HIV status of participants. The other inclusion criteria were fairly similar across all trials.
Interventions
Two trials (Kabir 1986; Rodriguez 1989) compared antibiotics and placebo or no drug. Both were three‐armed trials. Rodriguez 1989 compared furazolidone, cotrimoxazole, and no drug. Kabir 1986 compared ceftriaxone, ampicillin, and a placebo. Six trials compared flouroquinolones and beta‐lactams (Alam 1994, pivmecillinam and nalidixic acid; Bennish 1990, ciprofloxacin and ampicillin; Haltalin 1973, nalidixic acid and ampicillin; Leibovitz 2000, ciprofloxacin and ceftriaxone; Salam 1988, nalidixic acid and ampicillin; Salam 1998, ciprofloxacin and pivmecillinam). Two trials compared flouroquinolones and macrolides (Khan 1997a; Shanks 1999), both compared azithromycin and ciprofloxacin). Two trials compared cotrimoxazole and beta‐lactams (Prado 1993, pivmecillinam and cotrimoxazole; Nelson 1976a, cotrimoxazole and ampicillin). Gotuzzo 1989 compared cotrimoxazole and flouroquinolones (norfloxacin). Dutta 1995 compared furazolidone and nalidixic acid. Islam 1994 compared oral gentamicin and nalidixic acid. Bibile 1961 was a four‐armed trial: the first three had different types of sulphonamides: sulphamidine, sulphamethoxypyridazine, 'Streptotriad' and the fourth arm was tetracycline. Each tablet of Streptotriad contained streptomycin sulphate, sulphamerazine, sulphadiazine and sulphathiazole. This arm was not included in analysis (sulphonamide versus tetracycline) since it contained a non‐sulphonamide drug, streptomycin.
Outcomes
This review had two primary efficacy outcomes. The first primary outcome, diarrhoea on follow up, was reported by all but three trials (Kabir 1986; Gotuzzo 1989; Islam 1994); the duration of follow up was five days in 10/13 trials. The second primary outcome, relapse, was reported by four trials (Haltalin 1973; Salam 1998; Shanks 1999; Leibovitz 2000); the duration of follow up for this outcome ranged from 10 to 20 days. Among the secondary outcomes, fever at follow up was reported by four trials, time to cessation of fever was reported by five trials, time to cessation of diarrhoea was reported by six trials, time to cessation of blood in stools was reported by three trials, bacteriological cure or failure was reported by 11 trials, and development of severe complications was reported by only one trial. Duration of hospital stay was not an outcome measured by any of the trials. One trial (Kabir 1986) reported the mean number of stools per day in a graph that did not permit extraction of data for analysis. Adverse events were reported by all but four trials (Haltalin 1973; Alam 1994; Islam 1994; Dutta 1995). Only Leibovitz 2000 reported serious adverse events related to antibiotic therapy leading to hospitalization. None of the trials reported any deaths.
Excluded studies
We excluded 107 studies for the following reasons. Twenty‐nine studies were not RCTs. In 59 studies the inclusion criteria for the participants was not dysentery. Eighteen studies compared antibiotics of the same class, which we decided should be the subject of a separate review. One trial was excluded as the interventions were not antibiotics (Raqib 2008). Carbo 1981 awaits assessment as it provided no data on the numbers allotted to interventions and we are awaiting a reply from the authors.
Risk of bias in included studies
See Figure 1 for a summary of the 'risk of bias' in each included study and Figure 2 for a summary graph of methodological quality expressed as percentages across included trials. The risk of bias for each study is summarized additionally in 'Characteristics of included studies'.
Allocation
Among the included studies, 81% (13/16) had low risk of bias in the generation of the allocation sequence. Of these, four trials (Bibile 1961; Prado 1993; Salam 1998; Leibovitz 2000) used random number lists. The remaining trials (Nelson 1976a; Kabir 1986; Salam 1988; Gotuzzo 1989; Bennish 1990; Alam 1994; Islam 1994; Dutta 1995; Khan 1997a) used block randomization techniques. However, only 9/16 (56%) of the studies clearly reported adequate concealment of allocation (Salam 1988; Bennish 1990; Prado 1993; Alam 1994; Islam 1994; Dutta 1995; Khan 1997a; Salam 1998; Leibovitz 2000).
Blinding
Eleven trials (69%) had low risk of bias for the component of blinding. Salam 1988, Khan 1997a, Salam 1998, Shanks 1999 and Leibovitz 2000 had blinded the participant, the provider, and the outcome assessor. Kabir 1986, Bennish 1990, Prado 1993, Alam 1994 and Islam 1994 had blinded the participant and the provider. Dutta 1995 had only the outcome assessor blinded. Bibile 1961, Haltalin 1973, Nelson 1976a, Gotuzzo 1989 and Rodriguez 1989 were open trials.
Incomplete outcome data
Only 25% (4/16) trials (Bibile 1961; Haltalin 1973; Nelson 1976a; Kabir 1986) were judged to have adequately addressed incomplete outcome data. The remaining 12 trials did not adequately address incomplete outcome data because they excluded participants from data analysis after randomization as their stool cultures were later negative for Shigella. This is a serious methodological flaw (see 'Potential biases in the review process').
Selective reporting
All the studies were free of selective reporting.
Other potential sources of bias
More than 90% (15/16) of the studies had no other potential sources of bias. One study (Rodriguez 1989) had a significant baseline imbalance as the participants in one of the study arms had fewer days of diarrhoea than the other arms.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Summary of findings for the main comparison. Antibiotic versus no drug or placebo for Shigella dysentery.
| Antibiotic versus no drug or placebo for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Mexico and Bangladesh Intervention: Antibiotic versus no drug or placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotic versus no drug or placebo | |||||
| Diarrhoea on follow up ‐ Furazolidone versus no drug clinical criteria Follow‐up: 6 days | 58 per 100 | 12 per 100 (5 to 28) | RR 0.21 (0.09 to 0.48) | 73 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | Antibiotic sensitivity of Shigella isolates not reported; Trial done in 1989 |
| Diarrhoea on follow up ‐ Cotrimoxazole versus no drug clinical criteria Follow‐up: 6 days | 58 per 100 | 17 per 100 (9 to 34) | RR 0.3 (0.15 to 0.59) | 76 (1 study) | ⊕⊝⊝⊝ very low1,2,4 | Same trial as above; had three arms |
| Relapse ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | The two trials for this comparison were too short in follow up duration (6‐7 days) to estimate relapses and none were reported. |
| Serious adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | None of the two trials for this comparison reported serious adverse events |
| Other adverse events clinical criteria Follow‐up: 7 days | 0 per 100 | 0 per 100 (0 to 0) | RR 1.43 (0.06 to 34.13) | 94 (1 study) | ⊕⊝⊝⊝ very low5,6,7 | Data from a three armed trial; only one non‐serious adverse event in the antibiotic arms and none in placebo arm |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Very serious limitations: The method of randomization was not described and there were baseline imbalances in duration of diarrhoea. Allocation concealment and blinding were not reported and this increases the risk of bias in the detection and reporting of some adverse events, though not for other primary outcomes that were objectively ascertained. 2 Serious indirectness: The single trial included only children and hence the evidence for effectiveness of antibiotics over no antibiotics in adults is uncertain. Though the trial did not exclude participants who were malnourished, it is unclear if any participant was malnourished. 3 No imprecision: Both limits of the point estimate of the trial indicated benefit with furazolidine over not receiving an antibiotic 4 No imprecision: Both limits of the point estimate showed appreciable benefit with cotrimoxazole over not receiving an antibiotic 5 Very serious limitations: Allocation was not concealed and there were baseline imbalances in antibiotic sensitivity to those allocated to ceftriaxone (100%) and ampicillin (80%) 6 Serious indirectness: The trial randomized only adults. The antibiotics assessed were ceftriaxone and ampicillin. 7 Very serious imprecision: The 95% CI of the point estimate of the trial includes appreciable risk of adverse events for antibiotics over placebo with no significant differences between interventions.
Summary of findings 2. Fluoroquinolones versus beta‐lactams for Shigella dysentery.
| Fluoroquinolones versus beta‐lactams for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Bangladesh (4 trials), Israel (1 trial), USA (1 trial) Intervention: Fluoroquinolones versus beta‐lactams | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Fluoroquinolones versus beta‐lactams | |||||
| Diarrhoea on follow up ‐ All trials clinical criteria Follow‐up: 5 to 180 days | 251 per 1000 | 259 per 1000 (113 to 595) | RR 1.03 (0.45 to 2.37) | 686 (6 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | One trial from 1973; four trials in the 1990s; only one trial after 2000. The fluoroquinolones evaluated were nalidixic acid, and ciprofloxacin and the beta‐lactams evaluated were ampicillin, (intra‐muscular) ceftriaxone and pivmecillinam. |
| Relapse ‐ All trials clinical criteria Follow‐up: 5 to 180 days | 70 per 1000 | 64 per 1000 (8 to 529) | RR 0.91 (0.11 to 7.55) | 357 (3 studies) | ⊕⊝⊝⊝ very low5,6,7,8 | One trial from 1973, one from 1990 and one from 2000. Only two reported relapse. |
| Serious adverse events clinical criteria Follow‐up: 16 to 21 days | 0 per 100 | 0 per 100 (0 to 0) | RR 10.9 (0.61 to 194.82) | 221 (1 study) | ⊕⊝⊝⊝ very low9,10,11 | Only seen in 4.5% of those allocated to fluoroquinolones and not in those given beta‐lactams |
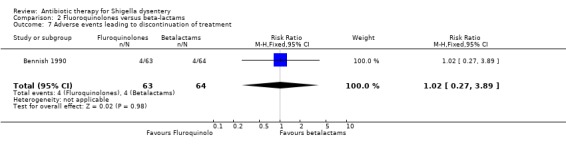
| Adverse events leading to discontinuation of treatment | 62 per 1000 | 64 per 1000 (17 to 245) | RR 1.02 (0.27 to 3.89) | 127 (1 study) | ⊕⊝⊝⊝ very low12,13,14 | |
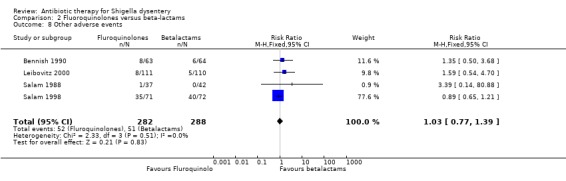
| Other adverse events clinical criteria Follow‐up: 5 to 180 days | 177 per 1000 | 182 per 1000 (136 to 246) | RR 1.03 (0.77 to 1.39) | 570 (4 studies) | ⊕⊝⊝⊝ very low15,16,17,18 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No serious limitations: Four of the six trials in this comparison had limitations in reporting outcomes for some participants but a sensitivity analysis did not appreciably alter the results 2 Serious inconsistency: I squared for the pooled data from six trials was 83% but could be partially explained by subgroup analyses of adults and children and by culture‐confirmed versus unconfirmed diagnosis of Shigella dysentery and resultant sensitivity patterns. The one trial in adults showed that a fluoroquinolone (ciprofloxacin) was superior (no imprecision) to a beta‐lactam (ampicillin) when sensitivity of the Shigella isolates was 100% for the former and 43% for the latter . Homogenous data (I squared 0%) from two trials in children showed that beta‐lactams (ampicillin and intra‐muscular ceftriaxone) were superior to fluoroquinolones (nalidixic acid and ciprofloxacin) when >90% of participants had culture‐confirmed Shigella dysentery with 100% sensitivity to the antibiotic used (no imprecision). 3 No serious indirectness: The six trials included children and adults and only two excluded severely malnourished children. The fluoroquinolones used included nalidixic acid and ciprofloxacin and the macrolides used included ampicillin, ceftriaxone and pivmecillinam. 4 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with both interventions. 5 No serious limitations: One of the three trials for this comparison had limitations in reporting the method of randomization and allocation concealment but exclusion of this trial in sensitivity analysis did not alter results. 6 Serious inconsistency: The I squared for the pooled data was 63% and could not explained by subgroup analyses. 7 Serious indirectness: The trials that reported this outcome only included children; hence the effects of antibiotics in preventing relapses in adults is unclear. 8 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with both interventions 9 No serious limitations: There were imbalances in those excluded from analysis in the single trial but randomization, allocation concealment and blinding were free of the risk of bias and follow up included 91% of participants 10 Serious indirectness: The trial included only infants and children and the applicability of the results for this outcome in adults is uncertain. 11 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with ceftriaxone and ciprofloxacin. 12 Serious limitations: This outcome was reported only for 75% of randomized participants with culture‐confirmed Shigella dysentery. 13 Serious indirectness: The trial that reported this outcome included only adults 14 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with ampicillin and ciprofloxacin in this single trial. 15 Serious limitations: Three of the four trials that reported this outcome reported on less than 85% of those randomised. 16 No inconsistency: I squared was 0% 17 No serious indirectness: The four trials included adults and children and two did not specifically exclude malnourished children. 18 Very serious imprecision: The 95% CI of the pooled estimate indicated appreciable harm and non‐appreciable benefit with beta‐lactams (ampicillin, ceftriaxone and pivmecillinam) over fluoroquinolones (ciprofloxacin and nalidixic acid)
Summary of findings 3. Fluoroquinolones versus macrolides for Shigella dysentery.
| Fluoroquinolones versus macrolides for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Bangladesh and Kenya Intervention: Fluoroquinolones versus macrolides | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Fluoroquinolones versus macrolides | |||||
| Diarrhoea on follow up clinical criteria Follow‐up: 6 to 10 days | 105 per 1000 | 63 per 1000 (25 to 156) | RR 0.6 (0.24 to 1.49) | 189 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | One trial reported that none of the participants had diarrhoea on day 10 and in the other 16/76 had diarrhoea on the sixth day |
| Relapse ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Duration of follow up in both trials were too short (6 to 10 days) to assess relapse and none were reported. |
| Serious adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | None of the two trials reported that any participant developed serious adverse events |
| Other adverse events clinical criteria Follow‐up: 6 days | Study population | RR 1.33 (0.32 to 5.56) | 76 (1 study) | ⊕⊝⊝⊝ very low5,6,7 | ||
| 79 per 1000 | 105 per 1000 (25 to 439) | |||||
| Medium risk population | ||||||
| 79 per 1000 | 105 per 1000 (25 to 439) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Serious limitations: One of the two included trials had limitations in allocation concealment and both reported outcomes for less than 90% of those randomized (82% and 87%) 2 No serious inconsistency: One of the trials had no participants with this outcome and hence risk ratios were estimated for only one trial. 3 Serious indirectness: Both trials randomized only adults. Effects of fluoroquinolones over macrolides in children, especially those who are malnourished are unclear. Antibiotics used were azithromycin and ciprofloxacin in both trials. 4 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with ciprofloxacin and azithromycin. 5 Very serious limitation: The trial reported this outcome only for 82% of randomized participants. 6 Serious indirectness: The trial included only adults. The antibiotics studied were ciprofloxacin and azithromycin. 7 Very serious imprecision:The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with ciprofloxacin and azithromycin.
Summary of findings 4. Cotrimoxazole versus beta‐lactams for Shigella dysentery.
| Cotrimoxazole versus beta‐lactams for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Guatemala and USA Intervention: Cotrimoxazole versus beta‐lactams | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cotrimoxazole versus beta‐lactams | |||||
| Diarrhoea on follow up clinical criteria Follow‐up: 11 to 21 days | 227 per 1000 | 134 per 1000 (52 to 338) | RR 0.59 (0.23 to 1.49) | 89 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4 | One trial was reported in 1976 and the other in 1993. The antibiotics compared with cotrimoxazole were ampicillin and pivmecillinam respectively. |
| Relapse ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | The two trials followed participants for 11 to 21 days but did not report any relapses in this time. |
| Serious adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No serious adverse events leading to death or hospitalization were reported in either trial. |
| Other adverse events clinical criteria Follow‐up: 11 to 21 days | 136 per 1000 | 110 per 1000 (37 to 333) | RR 0.81 (0.27 to 2.45) | 89 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Serious limitations: Inadequate allocation concealment in one trial and inadequate outcome data reporting (for 39% of randomized participants whose cultures were negative for Shigella) in the other 2 No inconsistency: I squared was 0% and the direction of effect favoured cotrimoxazole in both trials. 3 Serious indirectness: Both trials included only infants and children. The antibiotics compared were cotrimoxazole versus ampicillin and pivmecillinam. 4 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with cotrimoxazole and ampicillin and pivmecillinam. 5 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with beta‐lactams and cotrimoxazole.
Summary of findings 5. Cotrimoxazole versus fluoroquinolones (norfloxacin) for Shigella dysentery.
| Cotrimoxazole versus fluoroquinolones (norfloxacin) for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Peru Intervention: Cotrimoxazole versus fluoroquinolones (norfloxacin) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cotrimoxazole versus fluoroquinolones (norfloxacin) | |||||
| Diarrhoea on follow up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Outcome assessed as number of days to last unformed stool. Data not available for proportions with diarrhoea on follow up. |
| Relapse ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | The trial followed up participants for 14 days. Relapses were not reported in this time. |
| Serious adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No participant is reported to have developed serious adverse events leading to death or hospitalisation. |
| Other adverse events clinical criteria Follow‐up: 2 weeks | 0 per 1000 | 0 per 1000 (0 to 0) | RR 2.82 (0.12 to 66.62) | 62 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Very serious limitations: Inadequate allocation concealment and blinding and very inadequate outcome data reporting (for only 32% of 174 randomized). Baseline imbalance in antibiotic sensitivity (100% sensitivity in norfloxacin arm and 84% in the cotrimoxazole arm). 2 Serious indirectness: The trial included only adults. 3 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with cotrimoxazole and norfloxacin.
Summary of findings 6. Cotrimoxazole versus furazolidone for Shigella dysentery.
| Cotrimoxazole versus furazolidone for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Mexico Intervention: Cotrimoxazole versus furazolidone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cotrimoxazole versus furazolidone | |||||
| Diarrhoea on follow up clinical criteria Follow‐up: 6 days | 173 per 1000 | 123 per 1000 (47 to 318) | RR 0.71 (0.27 to 1.84) | 101 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | Trial reported in 1989; antimicrobial sensitivity to Shigella isolates not reported |
| Relapse ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Follow up duration too short (6 days) in the sole trial for this comparison |
| Serious adverse events | Medium risk population | RR 0 (0 to 0) | 0 (0) | See comment | No participant is reported to have developed serious adverse events leading to death or hospitalization. | |
| Other adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adverse effects reported; unclear if formally evaluated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Very serious limitations: Risk of bias likely due to inadequate allocation concealment and blinding. Baseline imbalances in participant characteristics (significantly fewer days of diarrhoea in those allocated to furazolidone‐ P =0.02). 2 Serious indirectness: The single trial included only infants and children. 3 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with cotrimoxazole over furazolidone.
Summary of findings 7. Oral gentamicin versus nalidixic acid for Shigella dysentery.
| Oral gentamicin versus nalidixic acid for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Bangladesh Intervention: Oral gentamicin versus nalidixic acid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oral gentamicin versus nalidixic acid | |||||
| Diarrhoea at follow up clinical criteria Follow‐up: 5 days | 308 per 1000 | 527 per 1000 (302 to 915) | RR 1.71 (0.98 to 2.97) | 79 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | Data from a single trial reported in 1994. Antimicrobial sensitivity for Shigella isolates was 100% in those allocated to oral gentamicin and 70% to those allocated to nalidixic acid. |
| Relapse ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Follow up duration too short (5 days) to assess. |
| Serious adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No participant is reported to have developed serious adverse events |
| Other adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No adverse effects reported; unclear if systematically assessed. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Very serious limitations: Though randomization, allocation and blinding were adequate, data were reported only for 87% randomized and there were baseline imbalances in antibiotic sensitivity (100% sensitive in gentamicin arm and 70% in nalidixic acid arm). 2 Serious indirectness: The trial randomized only infants and children and specifically excluded those severely malnourished. 3 Serious imprecision: The 95% CI for the point estimate from the trial includes appreciable and non‐appreciable benefit for nalidixic acid over oral gentamicin.
Summary of findings 8. Sulphonamides versus tetracycline for Shigella dysentery.
| Sulphonamides versus tetracycline for Shigella dysentery | ||||||
| Patient or population: patients with Shigella dysentery Settings: Sri Lanka Intervention: Sulphonamides versus tetracycline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Sulphonamides versus tetracycline | |||||
| Diarrhoea at follow up clinical criteria Follow‐up: 8 days | 0 per 1000 | 0 per 1000 (0 to 0) | RR 7.68 (0.46 to 128.12) | 60 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | Trial reported in 1961. Antimicrobial sensitivity not reported |
| Relapse ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Duration of follow up too short (8 days) to assess relapse |
| Serious adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | No participant is reported to have developed serious adverse events. |
| Other adverse events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported or pre‐stated as an outcome; unclear if assessed. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Very serious limitations: Risk of bias likely due to inadequate allocation concealment and blinding and unclear reporting of numbers randomized and numbers analysed. 2 Unclear indirectness: Unclear from report if trial included adults and children; malnourished participants were not specifically excluded. 3 Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and appreciable harm with tetracycline and sulphonamides.
We intended to prepare separate meta‐analyses for trials of: (1) an antibiotic drug versus another antibiotic drug belonging to the same or different drug class; (2) antibiotic drugs grouped by drug class versus other antibiotic drugs belonging to a different drug class; and (3) monotherapy with any antibiotic drug versus combination drug therapy with two or more different drugs given together or sequentially. However, we were only able to synthesize data from trials comparing single antibiotics of different classes and of antibiotics grouped by class. Comparisons of antibiotics within the same class were deferred to a subsequent review and thus 17 potential trials of this comparison were excluded from this review and are listed as such in the 'Characteristics of excluded studies'. We did not identify trials of an antibiotic drug versus combination drug therapy with two or more different drugs given together or sequentially.
We present trial results grouped as eight sets of comparisons.
1. Versus no drug or placebo (two trials)
Diarrhoea on follow up (primary outcome):
Rodriguez 1989 compared both oral furazolidone and cotrimoxazole with no treatment. Fewer patients in the antibiotic group had diarrhoea at follow up (for furazolidone, RR 0.21, 95% CI 0.09 to 0.48, 73 participants; and for cotrimoxazole versus no treatment, RR 0.30, 95% CI 0.15 to 0.59; 76 participants, Analysis 1.1).
1.1. Analysis.
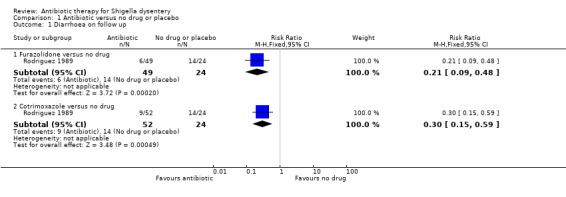
Comparison 1 Antibiotic versus no drug or placebo, Outcome 1 Diarrhoea on follow up.
Kabir 1986 compared intravenous ceftriaxone (n=64) and intravenous ampicillin (n=60) with placebo (n=30). There was no difference detected in time to diarrhoea resolution (Analysis 1.3), fever resolution (Analysis 1.2), and time to resolution of blood in the stools (Analysis 1.4), or adverse events (Analysis 1.5).
1.3. Analysis.
Comparison 1 Antibiotic versus no drug or placebo, Outcome 3 Time to cessation of diarrhoea (in days).
1.2. Analysis.
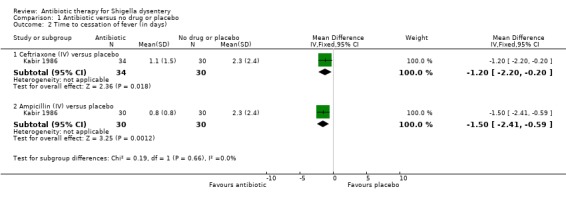
Comparison 1 Antibiotic versus no drug or placebo, Outcome 2 Time to cessation of fever (in days).
1.4. Analysis.
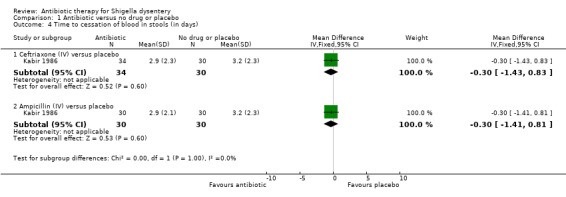
Comparison 1 Antibiotic versus no drug or placebo, Outcome 4 Time to cessation of blood in stools (in days).
1.5. Analysis.
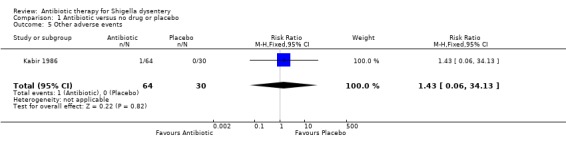
Comparison 1 Antibiotic versus no drug or placebo, Outcome 5 Other adverse events.
(See 'Table 1')
2. Fluoroquinolones versus beta‐lactams (six trials)
Diarrhoea on follow up (primary outcome):
Six trials measured this, and the comparative effects varied considerably between the trials, with no obvious trend (686 participants, six trials, Analysis 2.1; Haltalin 1973; Salam 1988; Bennish 1990; Alam 1994; Salam 1998; Leibovitz 2000). This variability was still present after exclusion of trials with a higher risk of bias (Haltalin 1973; Bennish 1990; Alam 1994; Salam 1988). Most of the trials were in children; one trial was in adults (Bennish 1990).
2.1. Analysis.
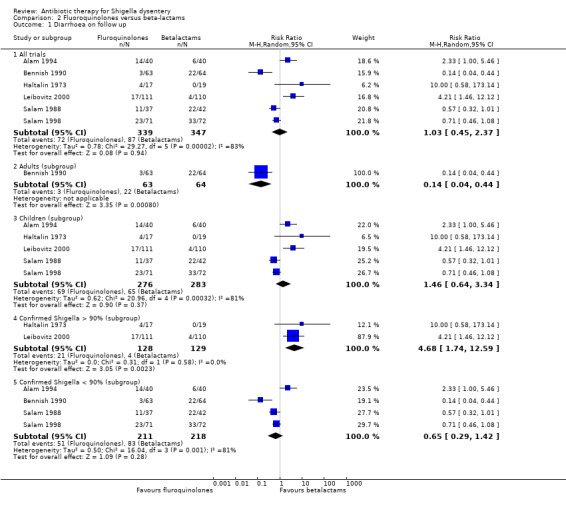
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 1 Diarrhoea on follow up.
In trials where 90% or more of included patients were confirmed with Shigella, beta‐lactams were more effective than fluoroquinolones (RR 4.68, 95% CI 1.74 to 12.59; 257 children, two trials, (Analysis 2.1). (Haltalin 1973; Leibovitz 2000); in the four trials with less than 90% confirmed Shigella positive patients the results showed no obvious pattern (Analysis 2.1). (Salam 1988; Bennish 1990; Alam 1994; Salam 1998).
Relapse:
No obvious pattern was apparent in the three trials examining this outcome ( Analysis 2.1; Haltalin 1973; Salam 1998; Leibovitz 2000) and subgroup analysis did not provide any further insights.
Fever at follow up:
Heterogenous data from two trials (Alam 1994; Salam 1998) showed no difference between the groups (191 participants, Analysis 2.2). Subgroup analysis was not done as both trials were done in children and had less than 90% of participants with Shigella in stool culture.
2.2. Analysis.
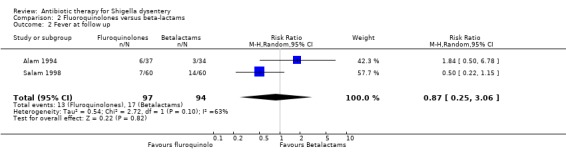
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 2 Fever at follow up.
Bacteriological failure:
Pooled heterogenous data from five trials (Haltalin 1973; Salam 1988; Bennish 1990; Alam 1994; Salam 1998) showed no difference between the two groups for this outcome (450 participants, Analysis 2.4). However on subgroup analysis based on participant's age, the single study done on adults (Bennish 1990) showed that fluoroquinolones were better than beta‐lactams in producing bacteriological cures (RR 0.28; 95% CI 0.08 to 0.95; 127 participants, Analysis 2.4). Even though the data from the children's subgroup (Haltalin 1973; Salam 1988; Alam 1994; Salam 1998) were homogenous, there was no difference between the two groups (223 participants, Analysis 2.4). The heterogeneity persisted on subgroup analysis based on number of participants with proven Shigella included in analysis.
2.4. Analysis.
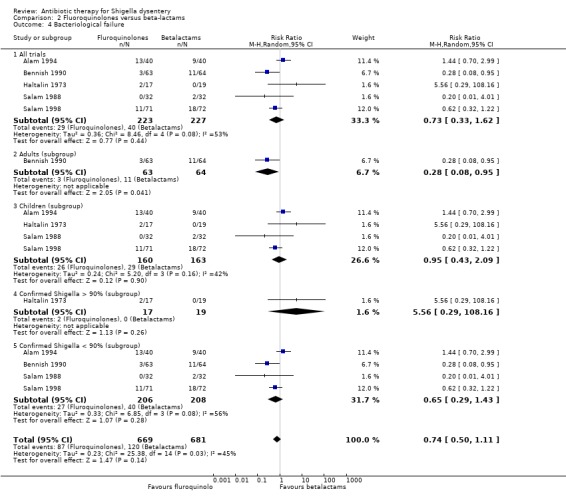
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 4 Bacteriological failure.
Development of severe complications:
Data from two trials (Haltalin 1973; Salam 1988) showed no difference between two groups for this outcome (90 participants, Analysis 2.5). Though formal tests did not reveal significant heterogeneity, the differences in size and direction of treatment effect for the two trials is important to consider in interpreting this result.
2.5. Analysis.
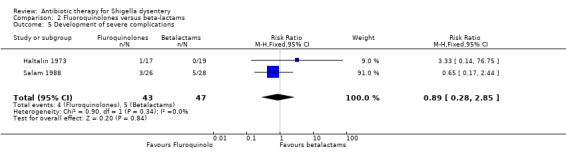
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 5 Development of severe complications.
Adverse events:
For serious adverse events,Leibovitz 2000 showed no difference between the two groups (Analysis 2.6, n=221); Bennish 1990 did not detect a difference in adverse events leading to discontinuation of treatment (127 participants, Analysis 2.7); for other adverse events, no difference was detected in four trials reporting this (Analysis 2.8). ( Salam 1988; Bennish 1990; Salam 1998; Leibovitz 2000).
2.6. Analysis.
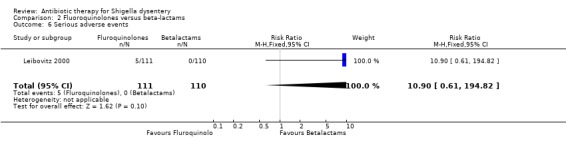
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 6 Serious adverse events.
2.7. Analysis.
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 7 Adverse events leading to discontinuation of treatment.
2.8. Analysis.
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 8 Other adverse events.
(See 'Table 2').
3. Fluoroquinolones versus macrolides (two trials)
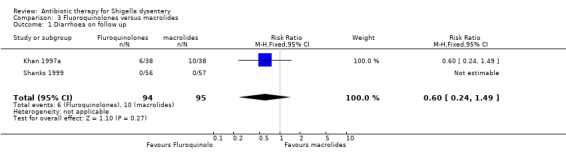
Diarrhoea on follow up (primary outcomes):
Data from two trials (Khan 1997a; Shanks 1999) showed no difference between the two groups (189 participants, Analysis 3.1). Heterogeneity could not be assessed since the results from Shanks 1999 were not estimable (no patients had diarrhoea on follow up in both arms) and hence neither subgroup analysis nor sensitivity analysis was done.
3.1. Analysis.
Comparison 3 Fluoroquinolones versus macrolides, Outcome 1 Diarrhoea on follow up.
Relapse:
Shanks 1999 reported on relapse but the results were not estimable as no patients had experienced relapse.
Fever at follow up:
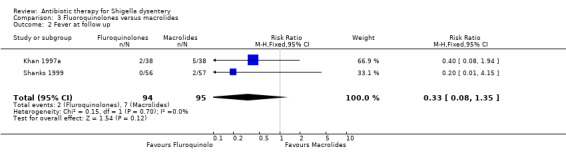
Homogenous data from two trials (Khan 1997a; Shanks 1999) showed no difference between the two groups (189 participants, Analysis 3.2).
3.2. Analysis.
Comparison 3 Fluoroquinolones versus macrolides, Outcome 2 Fever at follow up.
Time to cessation of blood in stool:
One trial (Shanks 1999) that reported this outcome showed no difference between the two groups (113 participants, Analysis 3.3).
3.3. Analysis.
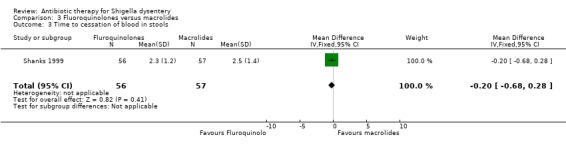
Comparison 3 Fluoroquinolones versus macrolides, Outcome 3 Time to cessation of blood in stools.
Bacteriological failure:
One trial (Khan 1997a) showed no difference between the two groups (76 participants, Analysis 3.4).
3.4. Analysis.
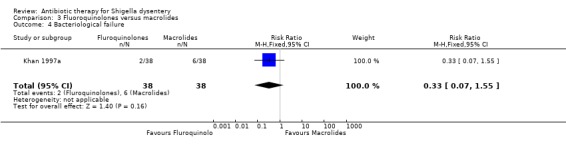
Comparison 3 Fluoroquinolones versus macrolides, Outcome 4 Bacteriological failure.
Adverse events:
Khan 1997a did not show any difference between the two groups (76 participants, Analysis 3.5).
3.5. Analysis.
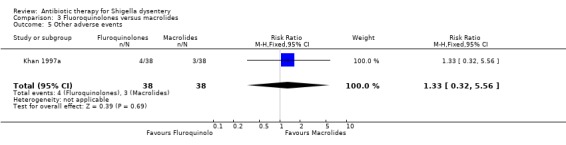
Comparison 3 Fluoroquinolones versus macrolides, Outcome 5 Other adverse events.
(See 'Table 3').
4. Cotrimoxazole versus beta‐lactams (two trials)
Diarrhoea on follow up (primary outcome):
Homogenous data from two trials (Nelson 1976a; Prado 1993) did not show any difference between the two groups (89 participants, Analysis 4.1). Exclusion of the poorer quality trial (Nelson 1976a) did not affect the results in sensitivity analysis.
4.1. Analysis.
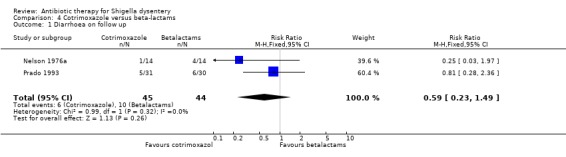
Comparison 4 Cotrimoxazole versus beta‐lactams, Outcome 1 Diarrhoea on follow up.
Bacteriological failure:
One trial (Nelson 1976a) which compared this outcome did not show any difference between two groups (28 participants, Analysis 4.2).
4.2. Analysis.
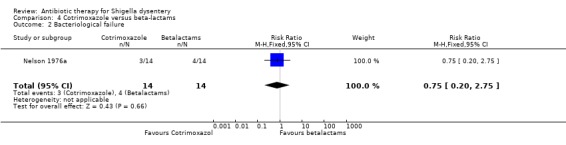
Comparison 4 Cotrimoxazole versus beta‐lactams, Outcome 2 Bacteriological failure.
Time to cessation of diarrhoea:
One trial (Prado 1993) that compared this outcome did not show any significant difference between the two groups (61 participants, Analysis 4.3).
4.3. Analysis.
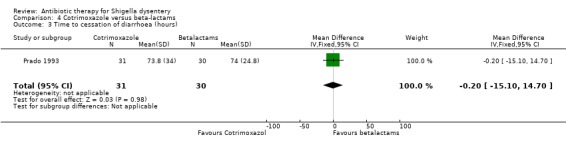
Comparison 4 Cotrimoxazole versus beta‐lactams, Outcome 3 Time to cessation of diarrhoea (hours).
Time to cessation of fever:
One trial (Prado 1993) reported this outcome and there was no difference between the two groups (61 participants, Analysis 4.4).
4.4. Analysis.
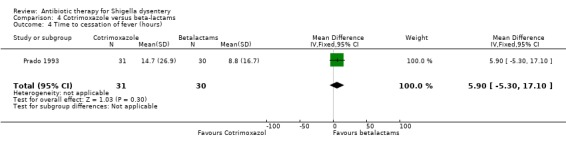
Comparison 4 Cotrimoxazole versus beta‐lactams, Outcome 4 Time to cessation of fever (hours).
Time to cessation of blood in stools:
One trial (Prado 1993) that compared this outcome did not show any difference between the two groups (61 participants, Analysis 4.5).
4.5. Analysis.
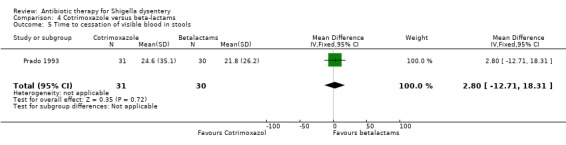
Comparison 4 Cotrimoxazole versus beta‐lactams, Outcome 5 Time to cessation of visible blood in stools.
Adverse events:
Homogenous data from two trials (Nelson 1976a; Prado 1993) showed no difference between the two groups for adverse events (89 participants, Analysis 4.6).
4.6. Analysis.
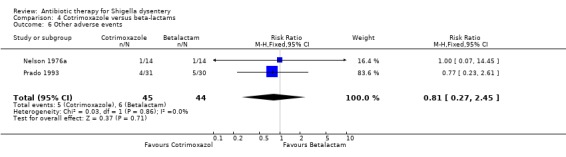
Comparison 4 Cotrimoxazole versus beta‐lactams, Outcome 6 Other adverse events.
(See 'Summary of findings table 4').
5. Cotrimoxazole versus fluoroquinolones (one trial)
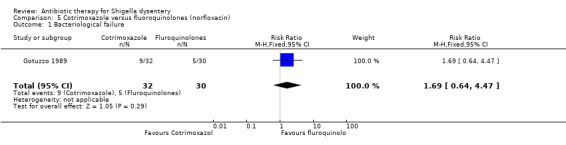
Bacteriological failure:
One trial (Gotuzzo 1989) that compared this outcome did not show any difference between the groups (62 participants, Analysis 5.1).
5.1. Analysis.
Comparison 5 Cotrimoxazole versus fluoroquinolones (norfloxacin), Outcome 1 Bacteriological failure.
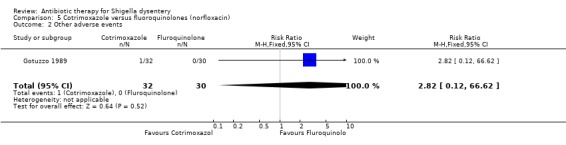
Adverse events:
Gotuzzo 1989, the only trial for this comparison, did not show any difference between the groups (62 participants, Analysis 5.2).
5.2. Analysis.
Comparison 5 Cotrimoxazole versus fluoroquinolones (norfloxacin), Outcome 2 Other adverse events.
(See 'Table 5').
6. Cotrimoxazole versus furazolidone (one trial)
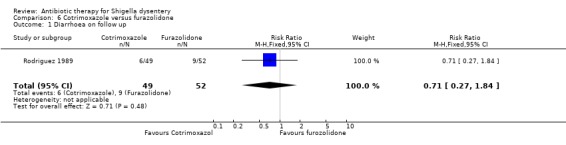
Diarrhoea on follow up (primary outcome):
One three‐armed trial (Rodriguez 1989, furazolidone, cotrimoxazole, and no drug) reported this outcome and there was no significant difference between the groups (101 participants, Analysis 6.1).
6.1. Analysis.
Comparison 6 Cotrimoxazole versus furazolidone, Outcome 1 Diarrhoea on follow up.
(See 'Table 6').
7. Oral gentamicin versus nalidixic acid (one trial)
Diarrhoea on follow up (primary outcome):
One trial (Islam 1994) that reported this outcome showed no difference between the two groups (79 participants, Analysis 7.1).
7.1. Analysis.
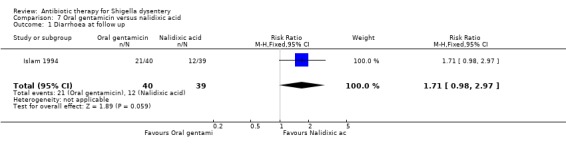
Comparison 7 Oral gentamicin versus nalidixic acid, Outcome 1 Diarrhoea at follow up.
Fever at follow up:
Islam 1994 reported this outcome and found nalidixic acid more effective than oral gentamicin in reducing the number patients with fever on follow up (RR 2.37, 95% CI 1.11 to 5.07; 79 participants, Analysis 7.2). While both the antibiotics were effective against Shigella in vitro, nalidixic acid was more effective in vivo due to better absorption when taken orally.
7.2. Analysis.
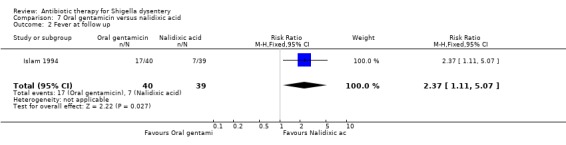
Comparison 7 Oral gentamicin versus nalidixic acid, Outcome 2 Fever at follow up.
Bacteriological failure:
Islam 1994 reported that nalidixic acid was more effective than oral gentamicin in achieving bacteriological cures (RR 2.10, 95% CI 1.29 to 3.42; 79 participants, Analysis 7.4).
7.4. Analysis.
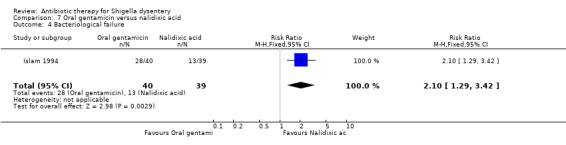
Comparison 7 Oral gentamicin versus nalidixic acid, Outcome 4 Bacteriological failure.
(See 'Summary of findings table 7')
8. Sulphonamides versus tetracyclines (one trial)
Diarrhoea on follow up (primary outcome):
One trial (Bibile 1961) that compared this outcome showed no difference between the two groups (60 participants, Analysis 8.1).
8.1. Analysis.
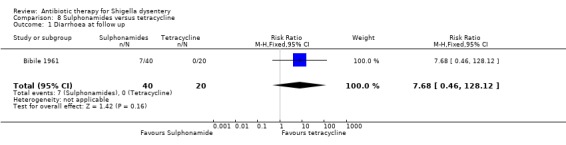
Comparison 8 Sulphonamides versus tetracycline, Outcome 1 Diarrhoea at follow up.
Bacteriological failure:
Bibile 1961 reported no difference between the groups (60 participants, Analysis 8.2).
8.2. Analysis.
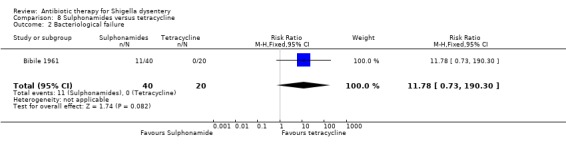
Comparison 8 Sulphonamides versus tetracycline, Outcome 2 Bacteriological failure.
(See 'Table 8').
Discussion
Summary of main results
This review identified 16 trials conducted over a span of four decades that randomized 1748 participants to evaluate the safety and efficacy of antibiotics in the treatment of Shigella dysentery. Most trials were at risk of bias due to limitations in reporting details of randomization or allocation concealment or blinding, but the most common source of bias occurred due to failure to report outcome details for participants who were randomized but in whom Shigella could not be isolated from stool culture.
In this review we focused on trials done with antibiotics belonging to different classes compared against placebo or no treatment or to each other. We found limited evidence to support the use of antibiotics in children and adults with Shigella dysentery compared to no treatment or placebo. One trial reported that antibiotics are effective in reducing the proportion of those with diarrhoea but it did not report on relapse. Another trial suggested that antibiotics were effective in reducing the duration of fever though they did not reduce the time to cessation of diarrhoea or bloody stool.
We did not find robust evidence to suggest that antibiotics of a particular class were better than those belonging to a different class. However, there were limited data from a subgroup of studies to suggest that a fluoroquinolone (ciprofloxacin) was more effective than a beta‐lactam (ampicillin) in reducing diarrhoea among adults and that beta‐lactams were more effective than fluoroquinolones in reducing diarrhoea among children with proven Shigella dysentery. Oral gentamicin was also reported to be inferior to nalidixic acid in achieving bacteriological cure and reducing fever in one small trial. The trials in this review report that at various periods of time different antibiotics have been effective against isolates of Shigella dysentery (Table 12) in different parts of the world. They are: ampicillin, cotrimoxazole, nalidixic acid, fluoroquinolones like ciprofloxacin, pivmecillinam, ceftriaxone, and azithromycin. However oral gentamicin was relatively ineffective, due to poor absorption when given orally, compared to nalidixic acid and therefore is not recommended. There was insufficient evidence to comment on the use of tetracyclines, sulphonamides, and chloramphenicol.
4. Sensitivity patterns of the Shigella isolates reported in included trials.
| Study ID | Group 1 | Group 2 | Group 3 |
| Alam 1994 |
Pivmecillinam group: All were sensitive to pivmecillinam Nalidixic acid sensitivity not reported |
Nalidixic acid group: All were sensitive to pivmecillinam 26/37, 45%, were sensitive to nalidixic acid |
Nil |
| Bennish 1990 |
Ciprofloxacin group: All were sensitive to ciprofloxacin; 34/60, 56.6%, were sensitive to ampicillin |
Ampicillin group: All were sensitive to ciprofloxacin; 26/61, 42.6%, were sensitive to ampicillin. |
Nil |
| Bibile 1961 | This is a 4‐armed trial with sulphadimidine, sulpha methoxy pyridazine, Strepto triad, and tetracycline in each group respectively Sensitivity patterns not reported for any group |
— | — |
| Dutta 1995 | Furazolidone and nalidixic acid Sensitivity patterns not reported for any group |
— | — |
| Gotuzzo 1989 |
Cotrimoxazole group: 27/32, 84%, were sensitive to cotrimoxazole; all were sensitive to norfloxacin |
Norfloxacin group: 26/30, 86%, were sensitive to cotrimoxazole; all were sensitive to norfloxacin |
Nil |
| Haltalin 1973 |
Nalidixic acid group: All were sensitive to nalidixic acid; ampicillin sensitivity not reported. |
Ampicillin group: All were sensitive to ampicillin; nalidixic acid sensitivity not reported |
Nil |
| Islam 1994 |
Nalidixic acid group: 26/37, 70%, were sensitive to nalidixic acid; all were sensitive to gentamicin |
Oral gentamicin: Nalidixic acid sensitivity not reported; all were sensitive to gentamicin |
Nil |
| Kabir 1986 |
Ceftriaxone group: All were sensitive to ceftriaxone; all were sensitive to ampicillin |
Ampicillin group: All were sensitive to ceftriaxone; 24/30, 80%, were sensitive to ampicillin |
Placebo: All were sensitive to ceftriaxone; 28/30, 93%, were sensitive to ampicillin |
| Khan 1997a |
Azithromycin group: All were sensitive to both antibiotics |
Ciprofloxacin group: All were sensitive to both antibiotics |
Nil |
| Leibovitz 2000 |
Ciprofloxacin group: All were sensitive to both antibiotics |
Ceftriaxone group: All were sensitive to both antibiotics |
Nil |
| Nelson 1976a | Cotrimoxazole group: All were sensitive to cotrimoxazole; 9/14, 64%, were sensitive to ampicillin |
Ampicillin group: All were sensitive to cotrimoxazole 10/14, 71%, were sensitive to ampicillin |
Nil |
| Prado 1993 |
Cotrimoxazole group; 24/30, 80%, were sensitive to cotrimoxazole; 25/30, 83.3%, were sensitive to pivmecillinam |
Pivmecillinam group: 23/29, 79.3%, were sensitive to cotrimoxazole; 26/29, 89.7%, were sensitive to pivmecillinam |
Nil |
| Rodriguez 1989 | 3‐armed trial with furazolidone, cotrimoxazole and a control (no antimicrobials) respectively Sensitivity patterns not reported for any group |
— | Nil |
| Salam 1988 |
Nalidixic acid group: All were sensitive to nalidixic acid; ampicillin sensitivity not reported |
Ampicillin group: All were sensitive to nalidixic acid; 25/40, 62.5%, were sensitive to ampicillin |
Nil |
| Salam 1998 |
Ciprofloxacin group: All were sensitive to ciprofloxacin; 58/60, 96.7%, were sensitive to pivmecillinam |
Pivmecillinam group: All were sensitive to ciprofloxacin; 57/60, 95%, were sensitive to pivmecillinam |
Nil |
| Shanks 1999 | Azithromycin and ciprofloxacin Sensitivity patterns not reported for any group |
— | Nil |
Sensitivity patterns not reported by 4 trials (Bibile 1961; Rodriguez 1989; Dutta 1995; Shanks 1999).
There is also insufficient evidence to indicate that any antibiotic class prevents relapse of Shigella dysentery.
None of the antibiotics studied in the trials were associated with major adverse events that were drug related.
Overall completeness and applicability of evidence
With respect to the review's objectives, this review found limited evidence that antibiotics reduce diarrhoea and the duration of fever compared to no antibiotic. However, we are unable to recommend an antibiotic or an antibiotic class for the treatment of Shigella dysentery. The studies identified could not sufficiently address relapse. All the antibiotics studied in this review were safe.
The studies addressed both adults and children. However, populations at risk for complicated Shigella dysentery, such as HIV infected populations and malnourished children, were not included (or adequately represented) in the trials we identified.
In current practice, antibiotics are recommended and used in the treatment of Shigella dysentery. The conclusions of this review confirm these recommendations and current practice. However this review is unable to recommend a specific antibiotic or antibiotic group as universally effective for the treatment of Shigella dysentery.
Even though mild forms of Shigella dysentery are said to be self‐limiting, this review is unable to comment on the need for antibiotics in this group since the included trials did not grade patients with respect to the severity of illness.
This review did not include studies using drugs belonging to similar antibiotic classes. Another review is needed to study differences between antibiotics belonging to the same class and also between different antibiotic dosing schedules, and short‐course versus longer‐course therapy of the same antibiotic.
Quality of the evidence
The body of evidence identified does not allow a robust conclusion regarding the objectives of the review or strong recommendations regarding the choice of preferred antibiotics. Of the 16 trials (1748 participants) included in the review, most had methodological limitations including inadequate reporting of the generation of allocation sequence, inadequate allocation concealment, and lack of blinding. Many trials removed participants after randomization since they did not grow Shigella in their stool culture and had not reported their outcome. This is a serious methodological error. Most trials were thus graded of low or very low quality and further research may change the estimates of efficacy and our confidence in these estimates.
Potential biases in the review process
We selected trials that compared the efficacy and safety of antibiotics of different classes only and deferred inclusion of trials evaluating antibiotics of the same drug class to an update or a separate review. Seventeen trials were excluded on the basis of this. This might have biased the results and conclusions of this review. We also did not include comparisons of different doses, routes of administration, or duration of treatment of the same antibiotic in Shigella dysentery.
We selected trials which included participants with clinical evidence of dysentery. However, Shigella infection can also present as diarrhoea in up to three‐quarters of infections, particularly in Asian countries (von Seidlein 2006). Excluding such patients in trials of antibiotics in Shigella and excluding trials using a broader definition than that used in this review could have biased the evidence presented. Many trials in this review also excluded participants randomized to receive antibiotics if their stool did not grow Shigella isolates. However, Shigella species and strains are highly sensitive to inhospitable environments and failure to grow Shigella in culture does not rule out Shigella infection (von Seidlein 2006). None of the included trials utilized alternative or additional, sensitive, diagnostic techniques such as identification of Shigella DNA using real‐time PCR. Exclusion of data from such participants in these trials, and exclusion of more stringent inclusion criteria for the diagnosis of Shigella dysentery in this review is likely to have introduced reporting and selection biases, respectively.
Agreements and disagreements with other studies or reviews
The overall results of this review suggest that most of the antibiotics used were effective. However, only 10 of the 16 included trials reported the proportion of participants that were sensitive to the antibiotics used. The outcomes in these trials correlated with the sensitivity patterns of the antibiotics used.
The WHO recommended nalidixic acid as the first line treatment for Shigella dysentery until 2004 when complete resistance to nalidixic acid in large parts of China and Bangladesh led to recommendations by the WHO to avoid using nalidixic acid altogether in Shigella dysentery (Legros 2004; WHO 2005a). However, nalidixic acid continues to be a potential option in parts of the world where resistance to this drug is not, as yet, a widespread problem, such as the Dakar region of the Senegal, where resistance to ampicillin, chloramphenicol, tetracycline, and cotrimoxazole are common (Sire 2008). However, widespread use of nalidixic acid may increase resistance to ciprofloxacin due to cross‐resistance of some strains of Shigella and thus has limited utility (WHO 2005a). The WHO recommends the use of ciprofloxacin as the first line antibiotic in suspected Shigella dysentery but also suggests that this choice should be based on sensitivity patterns of Shigella strains recently isolated in the area (WHO 2005a). Temporal and geographical shifts in Shigella strains are reported in parts of the world (von Seidlein 2006) and regular surveillance and ascertainment of antimicrobial sensitivity to local and regional strains is necessary to determine the choice of antibiotic to be used as first line in Shigella dysentery. Emerging drug resistance to ciprofloxacin and second line drugs such as pivmecillinam, ceftriaxone, and azithromycin is increasingly being reported in many parts of the world, as is multiple‐drug resistance (Kosek 2008; Kuo 2008; Pazhani 2008). The results of this review provides systematically ascertained evidence that the most commonly used antibiotics are potentially effective against Shigella dysentery, provided the local species and strains of Shigella are susceptible. Regular, periodic antibiotic‐susceptibility testing of isolates is required to guide local empiric therapy for Shigella dysentery.
Authors' conclusions
Implications for practice.
We recommend the use of antibiotics for moderate to severe Shigella dysentery. The choice of antibiotic to use as first line against Shigella dysentery should be governed by periodically updated local antibiotic sensitivity patterns of Shigella isolates. Other supportive and preventive measures recommended by the WHO (WHO 2005a; WHO 2005b) should also be instituted along with antibiotics (eg health education and handwashing).
Implications for research.
Randomized controlled trials which adhere to the CONSORT guidelines (CONSORT 2008) are required to address many of the issues such as the need for antibiotics in mild Shigella dysentery, the class or classes of antibiotics best suited against Shigella in populations at risk of high case‐fatality such as malnourished children, older adults, patients presenting with serious complications due to shigellosis, and HIV infected individuals.
Trials should stratify participants according to severity of clinical presentation and report the effects of antibiotics separately for each group. Trials must report outcomes for all randomized participants including those with confirmed Shigella and those with negative culture. Antibiotic sensitivity patterns should also be studied and reported. Data regarding outcomes presented in graphs and pictures also need to be expressed in numbers. See Table 13 for the suggested features of a future trial.
5. Suggestions for a trial of antibiotic for Shigella dysentery.
| Methods | Participants | Interventions | Outcomes | Notes |
|
Allocation:
Centralized sequence generation with table of random numbers or computer generated lists Stratified by severity of illness Sequence concealed until interventions are assigned Blinding: Those recruiting and assigning participants, those administering the intervention, and those assessing the outcomes, must all be blind to the allocated group; the administered drugs have to be identical or a double dummy technique has to be used. Liquid medications have to be in similar looking bottles, identical in shape and weight; the medications must themselves be similar in colour and flavour. Duration: Minimum of 4 weeks after completion of therapy to assess relapse |
Entry criteria can be clinical dysentery, i.e. acute onset frequent loose stools with blood or mucus or both lasting for less than 72 hours and at least 3 stools per day. Other features, such as fever and tenesmus at presentation, have to be recorded but need not be necessary for inclusion into study. If it is possible to presumptively or decisively detect Shigella in stool before inclusion into study, it should be done. Real‐time PCR is a rapid but expensive method to diagnose Shigella early (Legros 2004). Sample size: (See Table 14). Age group: trials should be separately done for adults and children (less than 15 years of age) or at least presented separately if they are in the same trial. In children, infants must be a separate group. Setting: in‐ or out‐patients. The number of participants, if hospitalized for standardization of administration of the interventions, have to be reported separately from those hospitalized due to complications. Sex: men and women. Special groups (those who have higher risk of complications:
Exclusion criteria: Allergy to the drug studied; history of antibiotic use for this episode of illness in the previous 48 hours; pregnant and lactating women; clinical presence of another infection needing antimicrobials |
Others: placebos or probiotics to be studied only on those with no risk of complications and those who have mild illness |
Primary outcomes:
Secondary outcomes:
|
Once patients are randomized into the treatment groups, they should not be removed until final analysis. The trial author(s) must publish the outcome findings of the whole group first and then present data for those positive for Shigella by stool or rectal swab culture or PCR and those negative for Shigella. The data have to be presented according to the severity of illness the patients presented with. Antibiotic sensitivity patterns have to be reported for all antibiotics studied and in all groups Response to treatment stratified by in vitro antibiotic sensitivity also needs to be reported Drop‐outs: The patients who drop out after randomization due to loss of follow up, withdrawal from protocol or consent withdrawal etc have to be reported and accounted in the final analysis (intention‐to‐treat analysis). |
6. Sample size suggestions for trial of antibiotics in Shigella dysentery.
| SAMPLE SIZES |
Antibiotic versus no drug or placebo (placebo response at 45%) or Antibiotic versus another antibiotic |
| 1 sided α | 10% difference: 310 20% difference: 75 25% difference: 50 30% difference: 30 40% difference: 15 |
| 2 sided α | 10% difference: 390 20% difference: 95 25% difference: 60 30% difference: 40 40% difference: 20 |
1. The sample size required to detect the assumed difference in improvement or worsening with 80% power and 5% significance level using the outcome of 'diarrhoea at follow up' from this review using StatCalc 2006. 2. The sample size mentioned is for each arm of the study.
What's new
| Date | Event | Description |
|---|---|---|
| 6 July 2010 | New citation required but conclusions have not changed | Author requested a name change |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 6 November 2009 | New citation required but conclusions have not changed | The name of the first author was incorrectly entered at first publication. The review is republished with a new citation in order to correct this. No other changes were made. |
Acknowledgements
We acknowledge the support of Prathap Tharyan, Katherine Abba, Paul Garner, Sara Bhattacharji, Gagandeep Kang, and Thambu David Sudarsanam, at various stages of this review. This protocol and review are the product of workshops conducted by the South Asian Cochrane Network & Centre that were partly funded by the Effective Health Care Research Programme Consortium (with funds from the Department for International Development (DFID), UK). The views expressed are not necessarily those of DFID.
Data and analyses
Comparison 1. Antibiotic versus no drug or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea on follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Furazolidone versus no drug | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.48] |
| 1.2 Cotrimoxazole versus no drug | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.15, 0.59] |
| 2 Time to cessation of fever (in days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Ceftriaxone (IV) versus placebo | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐2.20, ‐0.20] |
| 2.2 Ampicillin (IV) versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐2.41, ‐0.59] |
| 3 Time to cessation of diarrhoea (in days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Ceftriaxone (IV) versus placebo | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.41, 0.81] |
| 3.2 Ampicillin (IV) versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.37, 0.77] |
| 4 Time to cessation of blood in stools (in days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Ceftriaxone (IV) versus placebo | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.43, 0.83] |
| 4.2 Ampicillin (IV) versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.41, 0.81] |
| 5 Other adverse events | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.06, 34.13] |
Comparison 2. Fluoroquinolones versus beta‐lactams.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea on follow up | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All trials | 6 | 686 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.45, 2.37] |
| 1.2 Adults (subgroup) | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.04, 0.44] |
| 1.3 Children (subgroup) | 5 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.64, 3.34] |
| 1.4 Confirmed Shigella > 90% (subgroup) | 2 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 4.68 [1.74, 12.59] |
| 1.5 Confirmed Shigella < 90% (subgroup) | 4 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.29, 1.42] |
| 2 Fever at follow up | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.25, 3.06] |
| 3 Relapse | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 All trials | 3 | 357 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.11, 7.55] |
| 3.2 Confirmed Shigella > 90% (subgroup) | 2 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.11, 7.55] |
| 3.3 Confirmed Shigella < 90% (subgroup) | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Bacteriological failure | 5 | 1350 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.11] |
| 4.1 All trials | 5 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.33, 1.62] |
| 4.2 Adults (subgroup) | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.08, 0.95] |
| 4.3 Children (subgroup) | 4 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.43, 2.09] |
| 4.4 Confirmed Shigella > 90% (subgroup) | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 5.56 [0.29, 108.16] |
| 4.5 Confirmed Shigella < 90% (subgroup) | 4 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.29, 1.43] |
| 5 Development of severe complications | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.28, 2.85] |
| 6 Serious adverse events | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.90 [0.61, 194.82] |
| 7 Adverse events leading to discontinuation of treatment | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.27, 3.89] |
| 8 Other adverse events | 4 | 570 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.77, 1.39] |
2.3. Analysis.
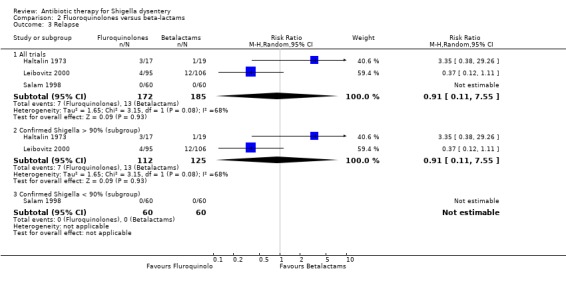
Comparison 2 Fluoroquinolones versus beta‐lactams, Outcome 3 Relapse.
Comparison 3. Fluoroquinolones versus macrolides.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea on follow up | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.24, 1.49] |
| 2 Fever at follow up | 2 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.08, 1.35] |
| 3 Time to cessation of blood in stools | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.68, 0.28] |
| 4 Bacteriological failure | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 5 Other adverse events | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.56] |
Comparison 4. Cotrimoxazole versus beta‐lactams.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea on follow up | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.23, 1.49] |
| 2 Bacteriological failure | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.20, 2.75] |
| 3 Time to cessation of diarrhoea (hours) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐15.10, 14.70] |
| 4 Time to cessation of fever (hours) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 5.90 [‐5.30, 17.10] |
| 5 Time to cessation of visible blood in stools | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐12.71, 18.31] |
| 6 Other adverse events | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.27, 2.45] |
Comparison 5. Cotrimoxazole versus fluoroquinolones (norfloxacin).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bacteriological failure | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.64, 4.47] |
| 2 Other adverse events | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.12, 66.62] |
Comparison 6. Cotrimoxazole versus furazolidone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea on follow up | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.84] |
Comparison 7. Oral gentamicin versus nalidixic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea at follow up | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.98, 2.97] |
| 2 Fever at follow up | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.11, 5.07] |
| 3 Bacteriological relapse | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.64, 5.95] |
| 4 Bacteriological failure | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.1 [1.29, 3.42] |
7.3. Analysis.
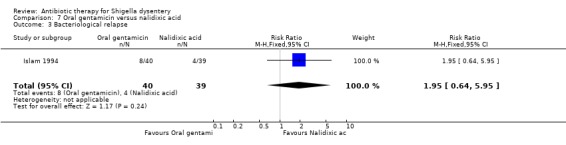
Comparison 7 Oral gentamicin versus nalidixic acid, Outcome 3 Bacteriological relapse.
Comparison 8. Sulphonamides versus tetracycline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea at follow up | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.68 [0.46, 128.12] |
| 2 Bacteriological failure | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.78 [0.73, 190.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alam 1994.
| Methods | Randomized controlled trial
Generation of allocation sequence: block randomization technique
Allocation concealment: drugs were stored in bottles, identical in appearance
Blinding: participants and provider blinded
Inclusion of all randomized participants: inadequate, 89% Duration: not mentioned |
|
| Participants | Number of participants enrolled: 80 Number of participants analysed: 71 Loss to follow up: none Inclusion criteria: children of both sexes between 1 and 8 years of age; having bloody diarrhoea lasting less than 72 hours Exclusion criteria: taken drugs for shigellosis; with systemic illnesses; severe malnutrition; | |
| Interventions | (1) Pivmecillinam (50 mg/kg/day, by mouth, in 4 divided doses, for 5 days) (2) Nalidixic acid (60 mg/kg/day, by mouth, in 4 divided doses, for 5 days) | |
| Outcomes | (1) Treatment failure (diarrhoea at follow up) by day 5
(2) Bacteriological failure on day 5
(3) Temperature > 37.8 ºC (fever on day 5) Not included in this review: (4) Abdominal pain or tenderness on day 5 |
|
| Notes | Location: Bangladesh Setting: all patients hospitalized in the study ward for the study period Follow‐up period: 6 days Antibiotic sensitivity pattern of Shigella isolates: 71/71, 100%, were sensitive to pivmecillinam; 26/37, 45%, in the nalidixic group sensitive to nalidixic acid. Nalidixic acid sensitivity is not reported in the pivmecillinam group. Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Block randomisation technique". Probably done. |
| Allocation concealment? | Low risk | "...patients were randomly allocated to treatment groups". There is no clear mention that allocation was concealed. Probably done as drugs were stored in serially numbered bottles (see below). |
| Blinding? All outcomes | Low risk | "Drugs were stored in bottles, identical in appearance, flavour and weight; labels on the bottles contained only the name of the study and the serial number of the patient who used the bottle." Participant and assessor blinding. |
| Incomplete outcome data addressed? All outcomes | High risk | 80 entered the study; 71 had Shigella in culture; no data regarding participants with non‐Shigella dysentery (9) who were randomized according to the inclusion criteria. Outcomes reported only for all 71 (89%) with culture confirmed Shigella dysentery. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Bennish 1990.
| Methods | Randomized controlled trial
Generation of allocation sequence: block randomization, random number table
Allocation concealment: medications and placebo packaged in identical appearing capsules
Blinding: participants, investigators, and assessor blinded
Inclusion of all randomized participants: inadequate, 75% Duration: 1 year and 3 months, from June 1986 to September 1987 |
|
| Participants | Number of participants enrolled: 161 Number of participants analysed: 121 Loss to follow up: 6 Inclusion criteria: dysentery less than 72 hours duration; adult males; age 18 to 60 years; no prior treatment with antimicrobial agent effective against shigellosis; absence of trophozoites of Entamoeba histolytica on stool microscopy Exclusion criteria: any other systemic illness additional to diarrhoea | |
| Interventions | (1) Ciprofloxacin (500 mg orally every 12 hours for 5 days) (2) Ampicillin (500 mg orally every 6 hours for 5 days) | |
| Outcomes | (1) On day 5, resolution of illness (patients with less than 3 stools, none watery, afebrile) (2) On day 5, marked improvement (patients with less than 6 stools, less than 1 watery stool) (3) On day 5, slight improvement (less than 9 stools, less than 2 watery stools) (4) On day 5, treatment failure (febrile, less than 10 stools, less than 3 watery stools) (5) Bacteriological cure (if Shigella species could not be cultured from a stool or rectal swab on study day 3 or after) (6) Mean stool frequency on day 3 (7) Adverse events (those that required discontinuation of the drug) (8) Other adverse events | |
| Notes | Location: Bangladesh Setting: all patients hospitalized in the study ward for 6 days after the first dose of medication Follow‐up period: 13 days Antibiotic sensitivity pattern of Shigella isolates: 121/121, 100%, were sensitive to ciprofloxacin; 34/60, 56.6%, in the ciprofloxacin group and 26/61, 42.6%, in the ampicillin group was sensitive to ampicillin Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomisation was done with block randomisation technique using a random number table and block size four". Probably done. |
| Allocation concealment? | Low risk | Not described but both drugs were identically packaged (see below); possibly concealed |
| Blinding? All outcomes | Low risk | "...both medications and placebo were packaged in identical‐appearing capsules, and patients, physicians, and nursing staff were blinded to their contents". Participant, investigator and assessor blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | Total randomized 161. Outcomes reported only for all 121 (75%) with culture confirmed Shigella dysentery. No data regarding participants with non‐Shigella dysentery (34) who were randomized according to the inclusion criteria. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Bibile 1961.
| Methods | Randomized controlled trial
Generation of allocation sequence: previously prepared list of random numbers
Allocation concealment: no
Blinding: not blinded
Inclusion of all randomized participants: unclear Duration: not mentioned |
|
| Participants | Number of participants enrolled: unclear Number of participants analysed: 80 Loss to follow up: unclear Inclusion criteria: 3 or more unformed stools per day with blood and mucus; tenesmus; no previous treatment; macroscopic and microscopic appearance of the stool comparable with bacillary not amoebic dysentery Exclusion criteria: amoebic dysentery | |
| Interventions | (1) Sulphadimidine (2 g immediately, followed by 1 g every 6 hours orally for 5 days)
(2) Sulpha methoxy pyridazine (1 g on first day and 0.5 g daily orally for a further 4 days)
(3)Tetracycline (250 mg orally every 6 hours for 5 days)
(4) "Strepto triad" (3 tablets three times daily, orally for 5 days; each tablet of streptotriad contains streptomycin 65 mg, sulphamerazine 65 mg, sulphadiazine 100 mg, and sulphathiazole 100 mg). This group was not included in the analysis (sulphonamides versus tetracycline) as it contains a non‐sulphonamide drug ‐ streptomycin. Other interventions: Injection pethidine given to one participant for severe tenesmus |
|
| Outcomes | (1) Number clinically cured by day 5 (2) Number bacteriologically cured (3) Mean duration of fever in days (4) Mean duration of abnormal stool in days | |
| Notes | Location: Sri Lanka Setting: not reported Follow‐up period: 8 days Antibiotic sensitivity pattern of Shigella isolates: not reported Funding source(s): Supplies of drugs from:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "...listed in a random order" |
| Allocation concealment? | High risk | "...previously prepared list of random numbers". Probably not done. |
| Blinding? All outcomes | High risk | Not mentioned; probably not done |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Dutta 1995.
| Methods | Randomized controlled trial
Generation of allocation sequence: random number table; permuted blocks of block length 8
Allocation concealment: sealed envelopes
Blinding: outcome assessor blinded; others unclear
Inclusion of all randomized participants: inadequate, 88% Duration: 8 months, from December 1992 to July 1993 |
|
| Participants | Number of participants enrolled: 72 Number of participants analysed: 63 Loss to follow up: 9 Inclusion criteria: children; both sexes; aged up to 5 years; with clinical diagnosis of dysentery (loose stool more than 3 times per day) Exclusion criteria: no prior antibiotic therapy, no systemic illness | |
| Interventions | (1) Furazolidone (7.5 mg/kg/day orally in 4 divided doses for 5 days) (2) Nalidixic acid (55 mg/kg/day orally in 4 divided doses for 5 days) | |
| Outcomes | (1) Clinical cure on day 3 and day 5 (no blood in stool, no fever, semisolid stools less than 3 times for last 24 hours, or no stool for last 18 hours) (2) Treatment failure on day 3 or day 5 (deterioration or no improvement in clinical parameters, for example fever, presence of blood, and mucus in stool or frequency of stool on day 5) | |
| Notes | Location: India Setting: participants were hospitalized during the trial period Follow‐up period: 5 days Antibiotic sensitivity pattern of Shigella isolates: not reported Funding source(s): none mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were randomised into two treatment groups...... in accordance with a random number table, using permuted block of block length eight" |
| Allocation concealment? | Low risk | "...sealed envelopes were used for treatment allocation" |
| Blinding? All outcomes | Low risk | "One of the investigators who had no knowledge of the drug administered monitored the clinical response"; only outcome assessor blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | "Two patients in furazolidone group and seven patients in the nalidixic acid group dropped out"; no reasons given. 87.4% follow up. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias. |
Gotuzzo 1989.
| Methods | Randomized controlled trial
Generation of allocation sequence: block randomization with a random number table
Allocation concealment: unclear
Blinding: nil
Inclusion of all randomized participants: inadequate, 32% Duration: not reported |
|
| Participants | Number of participants enrolled: 174 Number of participants analysed: 55 Loss to follow up: 7 Inclusion criteria: adults; dysentery; duration of illness less than 24 hours; informed consent Exclusion criteria: antibiotic therapy within 48 hours | |
| Interventions | (1) Cotrimoxazole (160/800 mg twice a day for 5 days) (2) Norfloxacin (800 mg single dose) | |
| Outcomes | (1) Days to last unformed stool (2) Number of culture positive follow up | |
| Notes | Location: Peru Setting: participants were not hospitalized but followed up in the out‐patients Follow‐up period: 2 weeks Antibiotic sensitivity pattern of Shigella isolates: 84% in the cotrimoxazole group and 86% in the norfloxacin group were sensitive to cotrimoxazole; 100% sensitivity in both groups to norfloxacin Funding source(s): in part by the International Collaboration in Infectious Disease Research grant 5 P01 A120130 from the National Institute of Allergy and Infectious Diseases |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Block randomization with a random number table |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | High risk | Not mentioned but unlikely to be blinded as the dosage regimens of interventions were different |
| Incomplete outcome data addressed? All outcomes | High risk | 174 entered the study; analysis was done on 55 (32%) patients; 62 had Shigella in culture; no data regarding participants with non‐Shigella dysentery (112) who were randomized according to the inclusion criteria. 7 patients were excluded from the culture Shigella positive 62 (5 from cotrimoxazole group due to drug resistance to the allocated drug and 2 others not mentioned). |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Haltalin 1973.
| Methods | Randomized controlled trial
Generation of allocation sequence: unclear
Allocation concealment: no
Blinding: unclear
Inclusion of all randomized participants: adequate, 100% Duration: not reported |
|
| Participants | Number of participants enrolled: 36 Number of participants analysed: 36 Loss to follow up: nil Inclusion criteria: infants and children; acute diarrhoeal disease; presumptive bacteriologic diagnosis of shigellosis; written informed consent from responsible legal guardian Exclusion criteria: infants under 1 month of age; known drug allergy; requiring specific antimicrobial therapy for concurrent infection | |
| Interventions | (1) Nalidixic acid (13.75 mg/kg, orally, every 6 hours for 5 days)
(2) Ampicillin (25 mg/kg, orally, every 6 hours for 5 days) Other interventions: Symptomatic treatment for fever and convulsions was ordered as necessary and was similar for both groups Fluid and electrolyte therapy and oral alimentation were given according to ward routine and was similar for both groups |
|
| Outcomes | (1) Number culture positive > 48 hours after start of treatment (2) Number culture positive > 5 days after start of treatment (3) Relapse (4) Number of days until culture negative (5) Diarrhoea > 5 days after start of treatment (6) Removed from protocol due to worsening (7) Number of days diarrhoea after start of treatment (8) Days until afebrile after start of treatment | |
| Notes | Location: United States of America Setting: hospital, in‐patient based trial Follow‐up period: 5 days Antibiotic sensitivity pattern of Shigella isolates: 17/17, 100%, in the nalidixic acid group were sensitive to nalidixic acid and 19/19, 100%, in the ampicillin group were sensitive to ampicillin. Nalidixic acid sensitivity in the ampicillin group and ampicillin sensitivity in the nalidixic group is not reported. Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "...randomly assigned"; but the method of sequencing not mentioned. In a previous trial done by the same author (Haltalin 1967) randomization was done based on the terminal digit number of the hospital record. The author could not be contacted for details since there was no mail ID. The journal's present editorial team did not have any details of the study. |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Islam 1994.
| Methods | Randomized controlled trial
Generation of allocation sequence: block randomization
Allocation concealment: was done by sequentially numbered identical containers. "Test drug and the standard drug were packed in identical bottles, were identical in appearance, flavour, and weight; the label of the bottles contained only the name of the study and the serial number of the patient for whom the bottle was used".
Blinding: participant and provider blinded
Inclusion of all randomized participants: inadequate, 89% Duration: 2 years, from January 1989 to December 1990 |
|
| Participants | Number of participants enrolled: 79 Number of participants analysed: 69 Loss to follow up: 10 Inclusion criteria: children between 1 and 8 years; bloody diarrhoea; duration of illness, less than 72 hours; absence of trophozoites of E. histolytica; with informed consent Exclusion criteria: systemic illness; severe malnutrition; taken effective anti‐Shigella drugs before coming to hospital | |
| Interventions | (1) Gentamicin (30 mg/kg, orally in 4 divided doses for 5 days) (2) Nalidixic acid (60 mg/kg, orally in 4 divided doses for 5 days) | |
| Outcomes | (1) Temperature > 37.8 ºC on post treatment days 1 (2) Temperature > 37.8 ºC on post treatment days 3 (3) Temperature > 37.8 ºC on post treatment days 5 (4) Isolation rates of Shigella species from stool/rectal swabs on post treatment days 1 (5) Isolation rates of Shigella species from stool/rectal swabs on post treatment days 2 (6) Isolation rates of Shigella species from stool/rectal swabs on post treatment days 3 (7) Isolation rates of Shigella species from stool/rectal swabs on post treatment days 4 (8) Isolation rates of Shigella species from stool/rectal swabs on post treatment days 5 (9) Bacteriologic relapse (10) Lack of clinical improvement (11) Lack of bacteriologic cure | |
| Notes | Location: Bangladesh Setting: participants were admitted in the study ward during the follow‐up period Follow‐up period: 5 days Antibiotic sensitivity pattern of Shigella isolates: all in both groups were sensitive to gentamicin; 26/37, 70%, in the nalidixic acid group were sensitive to nalidixic acid. Nalidixic acid sensitivity in the gentamicin group was not reported. Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "...randomly allocated to two treatment groups using a block randomisation technique." |
| Allocation concealment? | Low risk | "...packaged in identical bottles... The labels on the bottles contained only the name of the study and the serial number of the patient for whom the bottle was used" |
| Blinding? All outcomes | Low risk | Participant and provider |
| Incomplete outcome data addressed? All outcomes | High risk | 7/40 missing from the gentamicin group (5 failed to grow Shigella species; 1 developed severe broncho pneumonia and another required blood transfusion for severe anaemia and were excluded from the study); 3/39 missing from nalidixic acid group since they failed to grow Shigella species); 87% follow up |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Kabir 1986.
| Methods | Randomized controlled trial
Generation of allocation sequence: random numbers table
Allocation concealment: no
Blinding: participant and provider blinded
Inclusion of all randomized participants: adequate, 100% Duration: not reported |
|
| Participants | Number of participants enrolled: 94 Number of participants analysed: 94 Loss to follow up: nil Inclusion criteria: adult males; dysentery duration of illness less than 48 hours, more than 20 fecal leukocytes per high powered field; no trophozoites of E. histolytica in stool Exclusion criteria: other illnesses; history of allergy to penicillin; history of recent antibiotic therapy | |
| Interventions | (1) Ceftriaxone (1 g, intravenous, single dose) (2) Ampicillin (4 g, intravenous, single dose) (3) Placebo | |
| Outcomes | (1) Mean duration in days of diarrhoea (2) Mean duration in days of blood in stool (3) Mean duration in days of fever (4) Mean duration in days of positive stool culture | |
| Notes | Location: Bangladesh Setting: patients were requested to stay in the hospital for 7 days Follow‐up period: 7 days Antibiotic sensitivity pattern of Shigella isolates: all were sensitive to ceftriaxone; 34/34, 100%, in the ceftriaxone group, 24/30, 80%, in the ampicillin group and 28/30, 93%, in the placebo group were sensitive to ampicillin Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "...randomly allocated" |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Participants and provider blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data. Outcomes reported for all 94 with culture confirmed Shigella dysentery. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Khan 1997a.
| Methods | Randomized controlled trial
Generation of allocation sequence: random number table; block randomization with a block size of 6
Allocation concealment: adequate; the randomization list was developed and kept by a person not involved in the care or evaluation or in data analysis
Blinding: participant, provider and outcome assessor blinded
Inclusion of all randomized participants: inadequate, 83% Duration: not reported |
|
| Participants | Number of participants enrolled: 85 Number of participants analysed: 70 Loss to follow up: 6 Inclusion criteria: adult men aged 18 to 60 years; grossly bloody‐mucoid stool, tenesmus; duration of illness less than 72 hours; informed consent Exclusion criteria: taken an effective antimicrobial agent for current illness; co‐existing illness requiring antimicrobial therapy; had trophozoites of E. histolytica | |
| Interventions | (1) Azithromycin (500 mg, orally on day 1 followed by 250 mg orally for next 4 days) (2) Ciprofloxacin (500 mg, orally, every 12 hours for 5 days) | |
| Outcomes | (1) Clinical failure (2) Bacteriologic failure (3) Fever > 24 hours | |
| Notes | Location: Bangladesh Setting: patients were asked to stay in the hospital for a period of 6 days Follow‐up period: 6 days Antibiotic sensitivity pattern of Shigella isolates: all were sensitive to both antibiotics in both groups Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "...patients were given a consecutive study number to which treatment had been randomly pre assigned by use of a random number table...block randomisation method with a block size six was used" |
| Allocation concealment? | Low risk | "...randomisation list was developed and kept by a person not involved study" |
| Blinding? All outcomes | Low risk | "...double dummy technique"; participants, provider and outcome assessor blinded |
| Incomplete outcome data addressed? All outcomes | High risk | 9/85 participants were excluded from analysis as their rectal swab cultures did not grow Shigella; further, 6 of the remaining 76 were removed due to withdrawal from study (4 in the azithromycin group and 2 in the ciprofloxacin group). 83% follow up. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Leibovitz 2000.
| Methods | Randomized controlled trial
Generation of allocation sequence: computer list of random numbers
Allocation concealment: the list of random numbers was created by a person uninvolved in the study
Blinding: participant, provider and outcome assessor blinded
Inclusion of all randomized participants: adequate, 91% Duration: 1 year and 6 months, from July 1996 to December 1997 |
|
| Participants | Number of participants enrolled: 221 Number of participants analysed: 201 Loss to follow up: 5 Inclusion criteria: ambulatory infants and children; 6 months to 11 years; community acquired; acute invasive diarrhoea; illness that started less than 7 days before enrolment; grossly bloody‐mucoid stools on examination; more than or equal to soft or liquid stools within the last 24 hours; temperature more than or equal to 38 ºC, more than 15 white blood cells/high‐power microscopic field; able to take oral medications Exclusion criteria: were unable to take oral drugs; were receiving antibiotic therapy for the current illness, unless clinical failure was documented; were receiving antimicrobial treatment for more than 3 days for a concomitant infectious disease; needed hospitalization; had a known previous history of renal impairment, liver damage, cardiac disease or seizures; had a known hypersensitivity to either of the study drugs | |
| Interventions | (1) Ciprofloxacin suspension (10 mg/kg, every 12 hours for 3 days + placebo intramuscular injection, one shot per day for 3 days) (2) Ceftriaxone (intramuscular injection, 50 mg/kg/day, once daily for 3 days, maximal dose of 1 g per day + placebo suspension, one dose every 12 hours for 3 days) | |
| Outcomes | (1) Failure at end of therapy (day 4 to 5) (2) Relapse at end of follow up (day 21 +/‐ 5) | |
| Notes | Location: Israel Setting: not reported Follow‐up period: 21 +/‐ 5 days Antibiotic sensitivity pattern of Shigella isolates: all were sensitive to both antibiotics Funding source(s): in part by Bayer Corp., USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were randomly assigned to one of the therapies according to the computerized list provided by Pharma clinical limited" |
| Allocation concealment? | Low risk | "The randomisation list was developed and kept by a person not involved in the care or evaluation of the patients or in data analysis" |
| Blinding? All outcomes | Low risk | Blinding was done by "double dummy technique". Participant, provider and outcome assessor blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | "Sixteen and four patients from the ciprofloxacin and ceftriaxone group respectively, were excluded from the efficacy analysis because they are withdrawn from the study before its completion". 91% follow up. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Nelson 1976a.
| Methods | Randomized controlled trial
Generation of allocation sequence: random number tables
Allocation concealment: no
Blinding: nil
Inclusion of all randomized participants: adequate, 100% Duration: not reported |
|
| Participants | Number of participants enrolled: 28 Number of participants analysed: 28 Loss to follow up: nil Inclusion criteria: infants and children, diarrhoeic form of shigellosis (abrupt onset with high fever, prostration followed by large volume watery stools containing mucus, no blood); dysenteric form of shigellosis (onset is less abrupt, with a 1‐ to 3‐day period of increasing loose stools with blood, abdominal cramps and tenesmus) Exclusion criteria: none reported | |
| Interventions | (1) Cotrimoxazole suspension (40 mg trimethoprim and 200 mg sulphamethoxazole in each 5 ml, by mouth 1.25 ml/kg, daily in 2 doses every 12 hours for 5 days, total 10 doses)
(2) Ampicillin trihydrate suspension, by mouth, 100 mg/kg/day in divided doses every 6 hours for 5 days, total 20 doses Other interventions: Fluid and electrolyte therapy and diet were given according to ward routine Drugs were used in the management of high fever or convulsions |
|
| Outcomes | (1) Culture positive after > 48 hours (2) Diarrhoea > 5 days (3) Number of days until diarrhoea stopped (4) Adverse events | |
| Notes | Location: United States of America Setting: participants were admitted in the hospital for 5 days and then discharged and followed up in the out‐patients Follow‐up period: 14 to 21 days Antibiotic sensitivity pattern of Shigella isolates: all in both groups were sensitive to cotrimoxazole; 10/14, 71%, in the ampicillin group and 9/14, 64%, in the cotrimoxazole group were sensitive to ampicillin Funding source(s): Hoffmann‐La Roche, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Assignment was made according to a list generated from random number tables" |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | High risk | Ampicillin was given 4 times a day and cotrimoxazole was given 2 times a day without dummies |
| Incomplete outcome data addressed? All outcomes | Low risk | No missing outcome data. All randomized participants were used in analysis. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Prado 1993.
| Methods | Randomized controlled trial
Generation of allocation sequence: randomization list
Allocation concealment: randomization list was kept with WHO, Geneva and was broken only after the study was completed
Blinding: participant, investigator, and outcome assessor blinded by double dummy technique
Inclusion of all randomized participants: inadequate, 40% Duration: 2 years and 3 months, from November 1989 to January 1992 |
|
| Participants | Number of participants enrolled: 150 Number of participants analysed: 59 Loss to follow up: 2 Inclusion criteria: acute diarrhoea less than 3 days; children, age range 6 months to 13 years; clinical syndrome of dysentery (visible blood in stools and presence of sheets of polymorphonuclear white cells on stool examination or acute diarrhoea (passage of 3 liquid motions within 24 hours) with the presence of polymorphonuclear white cells on stool microscopy); weight for height index above 70% Exclusion criteria: treatment with antibiotics within 2 days prior to entry into the study; any life threatening illness due to Shigella; any concurrent disease that required treatment with antibiotics other than the drugs being studied; known hypersensitivity to penicillin or cotrimoxazole; presence of trophozoites of Entamoeba histolytica in stools | |
| Interventions | (1) Pivmecillinam (40 mg/kg/day in 4 doses per day)
(2) Cotrimoxazole (40 mg/kg/day in 4 doses per day) Other interventions: Dehydration was corrected with orally administered fluids as recommended by WHO |
|
| Outcomes | (1) Treatment failure (2) Duration of diarrhoea (3) Duration of fever (4) Duration of grossly visible blood in stools (5) Duration of positive stool culture (6) Adverse events | |
| Notes | Location: Guatemala Setting: participants were hospitalized for 5 days and then followed up in the out‐patients Study period: 11 to 13 days Antibiotic sensitivity pattern of Shigella isolates: 26/29 in pivmecillinam group and 25/30 in the cotrimoxazole group were sensitive to pivmecillinam; 23/29 in the pivmecillinam group and 24/30 in the cotrimoxazole group were sensitive to cotrimoxazole Funding source(s): World Health Organization |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomisation list" |
| Allocation concealment? | Low risk | "...randomisation list was kept with WHO, Geneva and was broken only after the study was completed" |
| Blinding? All outcomes | Low risk | Participant and provider blinded by "double dummy technique" |
| Incomplete outcome data addressed? All outcomes | High risk | 59/150 (39%) of randomized participants were not included in the analysis as Shigella strains not isolated. 2 patients who withdrew from the study on first day of treatment were not included in the analysis. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Rodriguez 1989.
| Methods | Randomized controlled trial
Generation of allocation sequence: unclear
Allocation concealment: no
Blinding: nil
Inclusion of all randomized participants: adequate, 100% Duration: 1 year and 7 months, from January 1987 to July 1988 |
|
| Participants | Number of participants enrolled: 125
Number of participants analysed: 123
Loss to follow up: nil
Inclusion criteria: children, aged 2 months to 59 months; passage of 3 or more watery stools in the previous 24 hours; history of diarrhoea up to 5 days before admission; and polymorphonuclear leucocytes and blood in stool samples Exclusion criteria: received in the previous 48 hours any antimicrobials, antidiarrhoeals or any other drug capable of modifying the course of the disease; who had amoeba in stools; any severe concomitant disease; any intolerance to the drug; any known allergy to the study drugs |
|
| Interventions | (1) Furazolidone (7.5 mg/kg/day, in 4 equally divided doses) (2) Cotrimoxazole (Trimethoprim (8 mg/kg/day) + sulphamethoxazole (40 mg/kg/day)) in 2 equally divided doses (3) Control group (no antimicrobials) | |
| Outcomes | (1) Cure/treatment success (in initial culture positive cases it is defined as both clinical cure, absence of diarrhoea and alleviation of all signs and symptoms by day 3 plus a bacteriological cure, a negative stool culture; in initial culture negative patients only clinical cure on day 3) (2) Adverse events | |
| Notes | Location: Mexico Setting: out‐patient study Follow‐up period: 6 days Antibiotic sensitivity pattern of Shigella isolates: not reported Funding source(s): Norwich Eaton Pharmaceuticals, Inc. (a Proctor and Gamble company) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "...randomised into three groups" but the method not mentioned. Neither the author nor the journal could be contacted for clarifications. |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | High risk | "Single blind"; not mentioned which group was blinded; blinding of the dosage schedules of the trial drugs in the 3 arms not done |
| Incomplete outcome data addressed? All outcomes | High risk | "...two patients in the control group were voluntarily withdrawn from the study". They were not included in the analysis. 98% follow up. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | High risk | Baseline imbalance, patients in furazolidone group had fewer days with diarrhoea (P value < 0.02) |
Salam 1988.
| Methods | Randomized controlled trial
Generation of allocation sequence: random number table; block randomization with block size of 16
Allocation concealment: unclear in the published data but a personal communication from the author revealed that allocation concealment was done
Blinding: participant, provider, and outcome assessor blinded
Inclusion of all randomized participants: inadequate, 71% Duration: not reported |
|
| Participants | Number of participants enrolled: 90 Number of participants analysed: 64 Loss to follow up: 5 Inclusion criteria: age between 6 months and 12 years; history of blood, mucoid diarrhoea and a stool specimen that had grossly visible blood and mucus; illness duration less than 72 hours Exclusion criteria: severe malnutrition; with systemic illnesses in addition to shigellosis; who had received allopathic medications other than anti pyretics | |
| Interventions | (1) Nalidixic acid (55 mg/kg/day, in 4 equally divided doses for 5 days) (2) Ampicillin (100 mg/kg/day in 4 equally divided doses for 5 days) | |
| Outcomes | (1) Stool frequency (2) Clinical cure (3) Rectal prolapse (4) Fever (5) Bacteriological failure on day 3 (6) Bacteriological failure on day 6 (7) Adverse events | |
| Notes | Location: Bangladesh Setting: participants were hospitalized for 6 days Follow‐up period: 6 days Antibiotic sensitivity pattern of Shigella isolates: all in both groups were sensitive to nalidixic acid. 25/40 in the ampicillin group were sensitive to ampicillin. Ampicillin sensitivity in the nalidixic acid group is not reported. Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "...random number table and block randomisation method with block size of 16". |
| Allocation concealment? | Low risk | "...patients were randomly assigned to receive either nalidixic acid or ampicillin" but the concealment method was not mentioned in the published data. Personal communication from the author revealed that allocation concealment was done. The drug was administered to the participating children by the research ward nurses, and the investigators only knew the random number pre‐assigned to one of the 2 drugs, by the randomization process. |
| Blinding? All outcomes | Low risk | "...drugs were administered as syrups that had similar colour, consistency, and flavour, and the concentration of each drug was adjusted so that patients received the same volume... patients, staff and investigators were unaware of which drug was being given." |
| Incomplete outcome data addressed? All outcomes | High risk | "data were analysed only from patients with culture‐confirmed cases of shigellosis who remained in the study for at least 24 hours." 90 enrolled, 74 eligible for analysis, 64 analysed. 10 drop‐outs ‐ 6 withdrawn by their parents, reasons not provided, 4 withdrawn because of lack of clinical improvement. 82% follow up. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Salam 1998.
| Methods | Randomized controlled trial
Generation of allocation sequence: computer generated list of random numbers
Allocation concealment: allocated by Bayer AG Pharma and not available to researchers, double dummy technique
Blinding: participants, providers and outcome assessor blinded
Inclusion of all randomized participants: inadequate, 84% Duration: 1 year and 8 months, from August 1995 to March 1997 |
|
| Participants | Number of participants enrolled: 143 Number of participants analysed: 120 Loss to follow up: 10 Inclusion criteria: children aged 2 years to 15 years; dysentery (passage of grossly bloody‐mucoid stools for 72 hours or less); who had not received any antimicrobial treatment (agent known to be effective in vivo against shigellosis and active in vitro against the Shigella strain isolated from the patient); gave informed consent Exclusion criteria: co‐existing disorders that required antimicrobial therapy | |
| Interventions |
|
|
| Outcomes | (1) Clinical failure (if patient did not have persistent dysentery on day 3, and if on day 5 a patient had 6 stools or less, no bloody‐mucoid stools, no more than 1 watery stool and no fever) (2) Bacteriological failure (bacteriological success: if the initial Shigella species could not be identified in culture on day 3 or later) (3) Fever less than 24 hours (4) Number of patients with bloody‐mucoid stools more than 3 days (5) Relapse (6) Adverse event ‐ limp (one of the adverse reactions to the antibiotic therapy could be a LIMP on walking due to joint pain caused by the antibiotics) (7) All adverse events | |
| Notes | Location: Bangladesh Setting: participants were hospitalized for 6 days after the first dose and then discharged for follow up Follow‐up period: 180 days Antibiotic sensitivity pattern of Shigella isolates: all in both groups were sensitive to ciprofloxacin. 58/60, in the ciprofloxacin group and 57/60 in the pivmecillinam group were sensitive to pivmecillinam. Funding source(s):
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Drug allocation used a computer‐generated list of random numbers". |
| Allocation concealment? | Low risk | "...list of random numbers, which was not available to the researchers". |
| Blinding? All outcomes | Low risk | "...double dummy technique". Participant, provider and outcome assessor blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | 13/143 (6 in the ciprofloxacin group and 7 in the pivmecillinam group) were excluded from analysis because they were found not eligible (12 did not grow Shigella in their stool culture and 1 had taken nalidixic acid before study entry). Further 10 (5 in each group) withdrew before study completion. 84% follow up. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Shanks 1999.
| Methods | Randomized controlled trial
Generation of allocation sequence: unclear
Allocation concealment: not mentioned
Blinding: participants, providers and outcome assessor blinded; double dummy
Inclusion of all randomized participants: inadequate, 87% Duration: not reported |
|
| Participants | Number of participants enrolled: 137
Number of participants analysed: 113
Loss to follow up: 17
Inclusion criteria: adults; acute dysentery (visible blood on recently passed unformed stools); not receiving antibiotics likely to be effective against Shigella species; if female and not pregnant as confirmed by urine testing; able to take oral medications; no study drug allergy; no alternative cause for dysentery; informed consent Exclusion criteria: not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Location: Kenya Setting: participants were hospitalized for 3 days after the first dose and then discharged for follow up in out‐patients Follow‐up period: 10 days Antibiotic sensitivity pattern of Shigella isolates: not reported Funding source(s): none mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Volunteers were... randomised to receive...". Mentioned randomized but not how generated. Author could not be contacted via e‐mail. |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Participants, providers and outcome assessor blinded; double dummy |
| Incomplete outcome data addressed? All outcomes | High risk | 17/130 were withdrawn as they left the hospital before completion of the study drug regimen. 87% follow up. |
| Free of selective reporting? | Low risk | The study's prespecified outcomes, which were of interest in this review, have been reported |
| Free of other bias? | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aoki 1987 | Not dysentery |
| Aoki 1989 | Not dysentery |
| Ashkenazi 1993 | Not dysentery |
| Barada 1980 | Not dysentery |
| Bassily 1994 | Not dysentery |
| Basualdo 2003 | Not dysentery |
| Bennish 1992 | Same antibiotic in all arms; quinolone, ciprofloxacin; 3‐arm trial, 1 g single dose versus 1 g at admission and 2nd dose at 24 hours versus 500 mg twice daily for 5 days |
| Bezjak 1966 | Not a RCT |
| Bhattacharya 1991 | Same class of drugs in all arms; quinolones; norfloxacin versus nalidixic acid |
| Bhattacharya 1992 | Same class of drugs in all arms; quinolones; norfloxacin versus nalidixic acid |
| Bhattacharya 1997 | Same class of drugs in all arms; quinolones; norfloxacin versus nalidixic acid |
| Bogaerts 1985 | Not a RCT |
| Browne 1983 | Not dysentery |
| Brugel 1950 | Not a RCT |
| Butler 1993 | Not dysentery |
| Cabada 1992 | Not dysentery |
| Camacho 1989 | Not dysentery |
| CDC 2006 | Not a RCT |
| Chang 1977 | Not dysentery |
| de Olarte 1974 | Not dysentery |
| Dryden 1996 | Not dysentery |
| Dumitriu 1992 | Not dysentery |
| DuPont 1973 | Not dysentery |
| DuPont 1982 | Not dysentery |
| DuPont 1983 | Not dysentery |
| DuPont 1984 | Not dysentery |
| DuPont 1986 | Not dysentery |
| DuPont 1992 | Not dysentery |
| DuPont 1992a | Not dysentery |
| Ekwall 1984 | Not dysentery |
| Ericsson 1983 | Not dysentery |
| Ericsson 1992 | Not dysentery |
| Fakouhi 1971 | Not a RCT |
| Gendrel 1997 | Not a RCT |
| Gilman 1980 | Same antibiotic in all arms; beta‐lactams; ampicillin, high‐dose (150 mg/kg/day) versus low‐dose (50 mg/kg/day) |
| Gilman 1981 | Same antibiotic in all arms; beta‐lactam; ampicillin, single dose (150 mg/kg; 1 dose) versus multiple doses (150 mg/kg/day for 5 days) |
| Goodman 1990 | Not dysentery |
| Ha 2008 | Same class of drugs in all arms; quinolones; ciprofloxacin versus gatifloxacin |
| Haltalin 1967 | Not dysentery |
| Haltalin 1968 | Not dysentery |
| Haltalin 1968a | Not a RCT |
| Haltalin 1969 | Not a RCT |
| Haltalin 1972 | Not a RCT |
| Haltalin 1972a | Not a RCT |
| Han 1998 | Same class of drugs in all arms; quinolones; rufloxacin versus homefloxacin |
| Hansson 1981 | Not dysentery |
| Helvaci 1998 | Same class of drugs in all arms; beta‐lactam; cefixime versus ampicillin‐sulbactam |
| Hiraishi 1980 | Not dysentery |
| Imagawa 1988 | Not dysentery |
| Iushchuk 2007 | Not a RCT |
| Jiang 1994 | Not a RCT |
| Jiang 2000 | Not a RCT |
| Jinhua 1992 | Not a RCT |
| Kabir 1984 | Same class of drugs in all arms; beta‐lactam; pivmecillinam versus ampicillin |
| Legros 2004 | Not a RCT |
| Lexomboon 1972 | Not dysentery |
| Lionel 1969 | Same antibiotic in all arms; macrolide; tetracycline; single‐dose (2.5 g single‐dose) versus multiple doses (250 mg, 6‐hourly for 5 days) |
| Lolekha 1988 | Not dysentery |
| Lolekha 1991 | Not dysentery |
| Mabadeje 1974 | Not dysentery |
| Mahllooji 2004 | Not dysentery |
| Martin 2000 | Not dysentery |
| Matsuoka 1995 | Not a RCT |
| Miles 1977 | Not a RCT |
| Mol 1987 | Same class of drugs in all arms; quinolones, enoxacin versus nalidixic acid |
| Moolasart 1999 | Not dysentery |
| Morisawa 1970 | Not dysentery |
| Motohiro 1982 | Not dysentery |
| Nelson 1975 | Not dysentery |
| Nelson 1976 | Not dysentery |
| Nikorowitsch 1978 | Not a RCT |
| Oldfield 1987 | Not dysentery |
| Orenstein 1981 | Not dysentery |
| Ostrower 1979 | Not dysentery |
| Petruccelli 1992 | Not dysentery |
| Pichler 1986 | Not dysentery |
| Pichler 1987 | Not dysentery |
| Prado 1981 | Not dysentery |
| Prado 1992 | Not dysentery |
| Rabbani 1982 | Not a RCT |
| Rakhmanova 1996 | Not a RCT |
| Raqib 2008 | Not antibiotics |
| Rogerie 1986 | Not a RCT |
| Sagara 1993 | Not a RCT |
| Sagara 1994 | Not a RCT |
| Saito 1983 | Not dysentery |
| Saito 1984 | Not dysentery |
| Salam 1995 | Same class of drugs in all arms; beta‐lactams, cefixime versus pivamdinocillin |
| Salam 1999 | Not a RCT |
| Sepp 1995 | Not dysentery |
| Seto 1992 | Not dysentery |
| Soares 1994 | Same class of drugs in all arms; quinolones; ciprofloxacin, short course (2 days) versus long course (5 days) |
| Soares 1996 | Same class of drugs in all arms; quinolones; 3‐arm trial, ciprofloxacin versus lomefloxacin long course versus lomefloxacin short course |
| Study Group 2002 | Same antibiotic in all arms; quinolone; ciprofloxacin 15 mg/kg/every 12 hours, short course (3 days) versus standard course (5 days) |
| Tian 1986 | Not a RCT |
| Tong 1970 | Not dysentery |
| Varsano 1991 | Not dysentery |
| Vinh 2000 | Same class of drugs in all arms; quinolones, ofloxacin versus nalidixic acid |
| Wistrom 1992 | Not dysentery |
| Xiouying 1986 | Not a RCT |
| Yamamoto 1973 | Not dysentery |
| Ye 1990 | Not a RCT |
| Yin 1998 | Same class of drugs in all arms; beta‐lactams; ceftriaxone made in China versus ceftriaxone made outside China |
| Yunus 1982 | Not dysentery |
| Yuying 1995 | Not a RCT |
| Zhang 1991 | Not dysentery |
'Not dysentery' means that not all participants have blood or mucus or both in stools at randomization. RCT = randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Carbo 1981.
| Methods | Randomized controlled trial (used a "randomisation table") Allocation concealment: not described Blinding: not specified Inclusion of all randomized participants: not reported Duration: unclear |
| Participants | Number of participants enrolled: not reported Number of participants analysed: not reported Loss to follow up: unclear Inclusion criteria: children over 6 years of age (age limit not mentioned); symptoms and positive bacterial culture Exclusion criteria: prior renal or hepatic disease |
| Interventions | Ampicillin: variable doses according to body weight for 7 days; number allocated not reported Ro‐12‐2510: 2 tablets every 24 hours; duration unclear; number allocated not reported |
| Outcomes | Clinical failure Microbiological failure Relapse |
| Notes | No numerical data provided on number randomized to each arm or for outcomes Further details from author awaited |
Differences between protocol and review
We intended to analyse combinations of an antibiotic drug versus another antibiotic drug of the same class or different drug classes. Comparisons of antibiotics within the same class were deferred to a subsequent review and thus 17 potential trials of this comparison were excluded from this review. The protocol was developed using Review Manager (RevMan) 4.2 and the review using RevMan 5 (Review Manager 2008). We intended to assess methodological quality of included studies using the methods described in Juni 2001. However, since the introduction of RevMan 5 (Review Manager 2008), a more detailed assessment of the risk of bias in included trials was undertaken, reported in 'Risk of bias' tables for each trial and graphically summarized in Figure 1 and Figure 2. We also used the GRADE profiler, version 3.2 (GRADE 2004) to create 'Summary of findings' tables for the primary outcomes in the review.
Had continuous data been summarised using geometric means, we would have combined them on the log scale using the generic inverse variance method and reported them on the natural scale.
Had outcomes been reported both at baseline and at a follow up or at trial endpoints, we would have extracted both the mean change from baseline and the standard deviation of this mean for each treatment group. We would also have extracted the means and standard deviation at baseline and follow up in each treatment group. If the data had been reported using geometric means, we would have recorded this information and extracted a standard deviation on the log scale.
Had count data been reported in trials, we intended to extract the total number of events in each group and the total amount of person‐time at risk in each group. We also intended to record the total number of participants in each group. If this information was not available, we would have extracted alternative summary statistics such as rate ratios and confidence intervals if available. Again, if count data were presented as dichotomous outcomes, we would have extracted the number of participants in each intervention group and the number of participants in each intervention group who experienced at least one event. If count data were presented as continuous outcomes or as a time‐to‐event outcomes, we would have extracted the same information as outlined for continuous and time‐to‐event outcomes. Count data would have been compared using rate ratios when the total number of events in each group and the total amount of person‐time at risk in each group are available, or by relative risks or weighted mean difference had the data been presented in dichotomous or continuous forms respectively. Hazard ratios from survival data would have been combined on the log scale using the inverse variance method and presented on the natural scale.
Had time‐to‐event outcomes been reported, we would have extracted the estimates of the log hazard ratio and its standard error. If standard errors were not available we would have extracted alternative statistics such as CIs or P values.
Contributions of authors
PC conceived the review and drafted the protocol. KVD, SMJ, and SV helped develop the protocol. Two teams of authors (PC and KVD & SMJ and SV) independently selected trials, assessed quality, extracted and entered data. All authors analysed and interpreted results and wrote the final review.
Sources of support
Internal sources
Low Cost Effective Care Unit, Christian Medical College, Vellore, India.
South Asian Cochrane Network & Centre, Vellore, India.
External sources
Department for International Development (DFID), UK.
-
Indian Council of Medical Research, India.
For support and funding for the Prof. BV Moses and ICMR Advanced Centre for Research & Training in Evidence‐Informed Healthcare
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Alam 1994 {published data only}
- Alam AN, Islam MR, Hossain MS, Mahalanabis D, Hye HKMA. Comparison of pivmecillinam and nalidixic acid in the treatment of acute shigellosis in children. Scandinavian Journal of Gastroenterology 1994;29(4):313‐7. [DOI] [PubMed] [Google Scholar]
Bennish 1990 {published data only}
- Bennish ML, Salam MA, Haider R, Barza M. Therapy for shigellosis. II. Randomized, double‐blind comparison of ciprofloxacin and ampicillin. Journal of Infectious Diseases 1990;162(3):711‐6. [DOI] [PubMed] [Google Scholar]
Bibile 1961 {published data only}
- Bibile SW, Cooray MPM, Balasubramaniam K, Gulasekaram J. Comparative trial of drugs in bacillary dysentery. Journal of Tropical Medicine and Hygeine 1961;64:300‐2. [PubMed] [Google Scholar]
Dutta 1995 {published data only}
- Dutta P, Sett A, Sarkar A, Mitra U, Saha D, Manna B, et al. Comparative efficacy of furazolidone and nalidixic acid in the empirical treatment of acute invasive diarrhoea: randomised clinical trial. Indian Pediatrics 1995;32(1):13‐9. [PubMed] [Google Scholar]
Gotuzzo 1989 {published data only}
- Gotuzzo E, Oberhelman RA, Maguina C, Berry SJ, Yi A, Guzman M, et al. Comparison of single‐dose treatment with norfloxacin and standard 5‐day treatment with trimethoprim‐sulphamethoxazole for acute shigellosis in adults. Antimicrobial Agents and Chemotherapy 1989;33(7):1101‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haltalin 1973 {published data only (unpublished sought but not used)}
- Haltalin KC, Nelson JD, Kusmiesz HT. Comparative efficacy of nalidixic acid and ampicillin for severe shigellosis. Archives of Disease in Childhood 1973;48(4):305‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 1994 {published data only}
- Islam MR, Alam AN, Hossain, MS, Mahalanabis D, Hye HK. Double‐blind comparison of oral gentamicin and nalidixic acid in the treatment of acute shigellosis in children. Journal of Tropical Pediatrics 1994;40(6):320‐5. [DOI] [PubMed] [Google Scholar]
Kabir 1986 {published data only}
- Kabir I, Butler T, Khanam A. Comparative efficacies of single intravenous doses of ceftriaxone and ampicillin for shigellosis in a placebo‐controlled trial. Antimicrobial Agents and Chemotherapy 1986;29(4):645‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 1997a {published data only}
- Khan WA, Seas C, Dhar U, Salam MA, Bennish ML. Treatment of shigellosis: V. Comparison of Azithromycin and Ciprofloxacin: A Double‐Blind, Randomized, Controlled Trial. Annals of Internal Medicine 1997;126(9):697‐703. [DOI] [PubMed] [Google Scholar]
Leibovitz 2000 {published data only}
- Leibovitz E, Janco J, Piglansky L, Press J, Yagupsky P, Reinhart H, et al. Oral ciprofloxacin vs. intramuscular ceftriaxone as empiric treatment of acute invasive diarrhoea in children. Pediatric Infectious Disease Journal 2000;19(11):1060‐7. [DOI] [PubMed] [Google Scholar]
Nelson 1976a {published data only}
- Nelson JD, Kusmiesz H, Jackson LH, Woodman E. Trimethoprim‐sulfamethoxazole therapy for shigellosis. JAMA 1976;235(12):1239‐43. [PubMed] [Google Scholar]
Prado 1993 {published data only}
- Prado D, Liu H, Velasquez T, Cleary TG. Comparative efficacy of pivmecillinam and cotrimoxazole in acute shigellosis in children. Scandinavian Journal of Infectious Diseases 1993;25(6):713‐9. [DOI] [PubMed] [Google Scholar]
Rodriguez 1989 {published data only (unpublished sought but not used)}
- Rodriguez RS, Chavez AZ, Galindo E. A randomised, controlled, single‐blind study comparing furazolidone with trimethoprim‐sulphamethoxazole in the empirical treatment of acute invasive diarrhoea. Scandinavian Journal of Gastroenterology. Supplement 1989;169:47‐53. [DOI] [PubMed] [Google Scholar]
Salam 1988 {published and unpublished data}
- Salam MA, Bennish ML. Therapy for shigellosis. I. Randomised, double‐blind trial of nalidixic acid in childhood shigellosis. Journal of Pediatrics 1988;113(5):901‐7. [DOI] [PubMed] [Google Scholar]
Salam 1998 {published data only}
- Salam MA, Dhar U, Khan WA, Bennish ML. Randomised comparison of ciprofloxacin suspension and pivmecillinam for childhood shigellosis. Lancet 1998;352:522‐7. [DOI] [PubMed] [Google Scholar]
Shanks 1999 {published data only (unpublished sought but not used)}
- Shanks GD, Smoak BL, Aleman GM, Oundo J, Waiyaki PG, Dunne MW, et al. Single dose of azithromycin or three‐day course of ciprofloxacin as therapy for epidemic dysentery in Kenya. Clinical Infectious Diseases 1999;29(4):942‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aoki 1987 {published data only}
- Aoki T, Shimizu N, Tomizawa I, Takizawa Y, Matsubara Y, Seo T, et al. Comparison of clinical efficacy of norfloxacin (NFLX) and pipemidic acid (PPA) in the treatment of infectious enteritis by a double‐blind method. Journal of the Japanese Association for Infectious Diseases 1987;61(7):830‐48. [DOI] [PubMed] [Google Scholar]
Aoki 1989 {published data only}
- Aoki T, Shimizu N, Tomizawa I, Takizawa Y, Matsubara Y, Nitta Y, et al. Comparison of clinical efficacy of lomefloxacin (LFLX, NY‐198) and pipemidic acid (PPA) in the treatment of infectious enteritis by a double‐blind method. Journal of the Japanese Association for Infectious Diseases 1989;63(6):606‐22. [DOI] [PubMed] [Google Scholar]
Ashkenazi 1993 {published data only}
- Ashkenazi S, Amir J, Waisman Y, Rachmel A, Garty BZ, Samra Z, et al. A randomised, double‐blind study comparing cefixime and trimethoprim‐sulphamethoxazole in the treatment of childhood shigellosis. The Journal of Pediatrics 1993;123(5):817‐21. [ISSN: CN‐00096970] [DOI] [PubMed] [Google Scholar]
Barada 1980 {published data only}
- Barada FA, Guerrant RL. Sulfamethoxazole‐trimethoprim versus ampicillin in treatment of acute invasive diarrhoea in adults. Antimicrobial Agents and Chemotherapy 1980;17(6):961‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassily 1994 {published data only}
- Bassily S, Hyams K C, Masry NA, Farid Z, Cross E, Bourgeois AL, Ayad E, Hibbs RG. Short‐course norfloxacin and trimethoprim‐sulphamethoxazole treatment of shigellosis and salmonellosis in Egypt. American Journal of Tropical Medicine and Hygiene 1994;51(2):219‐23. [DOI] [PubMed] [Google Scholar]
Basualdo 2003 {published data only}
- Basualdo W, Arbo A. Randomized comparison of azithromycin versus cefixime for treatment of shigellosis in children. Pediatric Infectious Disease Journal 2003;22(4):374‐7. [PubMed] [Google Scholar]
Bennish 1992 {published data only}
- Bennish ML, Salam MA, Khan WA, Khan AM. Treatment of shigellosis: III. Comparison of one‐ or two‐dose ciprofloxacin with standard 5‐day therapy. A randomised, blinded trial. Annals of Internal Medicine 1992;117(9):727‐34. [DOI] [PubMed] [Google Scholar]
Bezjak 1966 {published data only}
- Bezjak B, Breitenfeld V. Therapy of shigellosis and chronic intestinal amoebiasis with enterosediv [Die Therapie der Shigellose und der chronischen Intestinal amoebiasis mit Enterosediv]. Therapie Der Gegenwart 1966;103(9):1129‐36. [PubMed] [Google Scholar]
Bhattacharya 1991 {published data only}
- Bhattacharya SK, Bhattacharya MK, Dutta P, Sen D, Rasaily R, Moitra A, et al. Randomized clinical trial of norfloxacin for shigellosis. American Journal of Tropical Medicine and Hygiene 1991;45(6):683‐7. [DOI] [PubMed] [Google Scholar]
Bhattacharya 1992 {published data only}
- Bhattacharya MK, Nair GB, Sen D, Paul M, Debnath A, Nag A, et al. Efficacy of norfloxacin for shigellosis: a double‐blind randomised clinical trial. Journal of Diarrhoeal Diseases Research 1992;10(3):146‐50. [PubMed] [Google Scholar]
Bhattacharya 1997 {published data only}
- Bhattacharya K, Bhattacharya MK, Dutta D, Dutta S, Deb M, Deb A, et al. Double‐blind, randomised clinical trial for safety and efficacy of norfloxacin for shigellosis in children. Acta Paediatrica 1997;86(3):319‐20. [DOI] [PubMed] [Google Scholar]
Bogaerts 1985 {published data only}
- Bogaerts J, Habyalimana JB, Chevolet T, Vandepitte J. Treatment of severe bacillary dysentery with trimethoprim alone. Transactions of the Royal Society of Tropical Medicine and Hygiene 1985;79(2):203‐5. [DOI] [PubMed] [Google Scholar]
Browne 1983 {published data only}
- Robins‐Browne RM, Coovadia HM, Bodasing MN, Mackenjee MKR. Treatment of acute nonspecific gastroenteritis of infants and young children with erythromycin. American Journal of Tropical Medicine and Hygiene. 1983;32(4):886‐90. [DOI] [PubMed] [Google Scholar]
Brugel 1950 {published data only}
- Brügel H. On the treatment of bacillary dysentery with phages specific for the endemic infection [Über die Behandlung der Bazillen‐Ruhr mit epidemiespezifischen Ruhrphagen]. Deutsche Medizinische Wochenschrift 1950;75(51):1751‐2. [DOI] [PubMed] [Google Scholar]
Butler 1993 {published data only}
- Butler T, Lolekha S, Rasidi C, Kadio A, Rosal PL, Iskandar H, et al. Treatment of acute bacterial diarrhoea: a multicenter international trial comparing placebo with fleroxacin given as a single dose or once daily for 3 days. American Journal of Medicine 1993;94(3A):187S‐194S. [PubMed] [Google Scholar]
Cabada 1992 {published data only}
- Cabada FJ, DuPont HL, Gyr K, Mathewson JJ. Antimicrobial therapy of bacterial diarrhoea in adult residents of Mexico ‐ lack of an effect. Digestion 1992;53(3‐4):134‐41. [DOI] [PubMed] [Google Scholar]
Camacho 1989 {published data only}
- Camacho JLP. A comparison of furazolidone and ampicillin in the treatment of invasive diarrhoea. Scandinavian Journal of Gastroenterology. 1989;16(Suppl):954‐9. [DOI] [PubMed] [Google Scholar]
CDC 2006 {published data only}
- Centers for Disease Control and Prevention. Update on emerging infections: news from the Centers for Disease Control and Prevention. Annals of Emergency Medicine 2006;47(1):106‐9. [DOI] [PubMed] [Google Scholar]
Chang 1977 {published data only}
- Chang MJ, Dunkle LM, Reken D, Anderson D, Wong ML, Feigin RD. Trimethoprim‐sulfamethoxazole compared to ampicillin in the treatment of shigellosis. Pediatrics 1977;59(5):726‐9. [PubMed] [Google Scholar]
de Olarte 1974 {published data only}
- Garcia de Olarte D, Trujillo H, Agudelo N, Nelson JD, Haltalin KC. Treatment of diarrhoea in malnourished infants and children. A double‐blind study comparing ampicillin and placebo. American Journal of Diseases of Children 1974;127(3):379‐88. [DOI] [PubMed] [Google Scholar]
Dryden 1996 {published data only}
- Dryden MS, Gabb RJ, Wright SK. Empirical treatment of severe acute community‐acquired gastroenteritis with ciprofloxacin. Clinical Infectious Diseases 1996;22(6):1019‐25. [DOI] [PubMed] [Google Scholar]
Dumitriu 1992 {published data only}
- Dumitriu S, Dumitriu M, Dimitriu SM, Turcu T, Grigore L, Dumitriu D, et al. Bioactive polymers: in vitro and in vivo study of controlled release neomycin. Journal of Biomaterials Applications 1992;6(3):251‐60. [DOI] [PubMed] [Google Scholar]
DuPont 1973 {published data only}
- DuPont HL, Hornick RB. Adverse effect of lomotil therapy in shigellosis. JAMA 1973;226(13):1525‐8. [PubMed] [Google Scholar]
DuPont 1982 {published data only}
- DuPont HL, Reves RR, Galindo E, Sullivan PS, Wood LV, Mendiola JG. Treatment of travellers' diarrhoea with trimethoprim/sulphamethoxazole and with trimethoprim alone. New England Journal of Medicine 1982;307(14):841‐4. [DOI] [PubMed] [Google Scholar]
DuPont 1983 {published data only}
- Dupont HL, Ericsson CD, Galindo E, Dupont MW, Mendiola Gomez J. Antimicrobial therapy of travellers' diarrhoea. Scandinavian Journal of Gastroenterology 1983;84(Suppl):99‐105. [PubMed] [Google Scholar]
DuPont 1984 {published data only}
- DuPont HL, Ericsson CD, Galindo E, Wood LV, Morgan D, Bitsura JA, et al. Furazolidone versus ampicillin in the treatment of traveller's diarrhoea. Antimicrobial Agents and Chemotherapy 1984;26(2):160‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
DuPont 1986 {published data only}
- DuPont HL, Ericsson CD, Reves RR, Galindo E. Antimicrobial therapy for travellers' diarrhoea. Reviews of Infectious Diseases 1986;8(Suppl 2):S217‐22. [DOI] [PubMed] [Google Scholar]
DuPont 1992 {published data only}
- DuPont HL, Ericsson CD, Mathewson JJ, DuPont MW. Five versus three days of ofloxacin therapy for traveller's diarrhoea: a placebo‐controlled study. Antimicrobial Agents and Chemotherapy 1992;36(1):87‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
DuPont 1992a {published data only}
- DuPont HL, Ericsson CD, Mathewson JJ, Cabada FJ, Conrad DA. Oral aztreonam, a poorly absorbed yet effective therapy for bacterial diarrhoea in US travellers to Mexico. JAMA 1992;267(14):1932‐5. [PubMed] [Google Scholar]
Ekwall 1984 {published data only}
- Ekwall E, Jonsson M. A comparison of the combination pivmecillinam/pivampicillin and co‐trimoxazole in the treatment of convalescent carriers of Salmonella and Shigella. Scandinavian Journal of Infectious Diseases 1984;16(1):99‐102. [DOI] [PubMed] [Google Scholar]
Ericsson 1983 {published data only}
- Ericsson CD, DuPont HL, Sullivan P, Galindo E, Evans DG, Evans DJJr. Bicozamycin, a poorly absorbable antibiotic, effectively treats travellers' diarrhoea. Annals of Internal Medicine 1983;98(1):20‐5. [DOI] [PubMed] [Google Scholar]
Ericsson 1992 {published data only}
- Ericsson CD, Nicholls‐Vasquez I, DuPont HL, Mathewson JJ. Optimal dosing of trimethoprim‐sulphamethoxazole when used with loperamide to treat traveller's diarrhoea. Antimicrobial Agents and Chemotherapy 1992;36(12):2821‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fakouhi 1971 {published data only}
- Fakouhi T, Post C, Dutz W. Clinical trial with sulphamethoxazole‐trimethoprim in infantile gastroenteritis. Current Therapeutic Research, Clinical and Experimental 1971;13(1):13‐7. [PubMed] [Google Scholar]
Gendrel 1997 {published data only}
- Gendrel D, Moreno JL, Nduwimana M, Baribwira C, Raymond J. One‐dose treatment with pefloxacin for infection due to multidrug‐resistant Shigella dysenteriae type I in Burundi. Clinical Infectious Diseases 1997;24(1):83. [DOI] [PubMed] [Google Scholar]
Gilman 1980 {published data only}
- Gilman RH, Koster F, Islam S, McLaughlin J, Rahaman MM. Randomized trial of high‐ and low‐dose ampicillin therapy for treatment of severe dysentery due to Shigella dysenteriae type 1. Antimicrobial Agents and Chemotherapy 1980;17(3):402‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilman 1981 {published data only}
- Gilman RH, Spira W, Rabbani H, Ahmed W, Islam A, Rahaman MM. Single‐dose ampicillin therapy for severe shigellosis in Bangladesh. Journal of Infectious Diseases 1981;143(2):164‐9. [DOI] [PubMed] [Google Scholar]
Goodman 1990 {published data only}
- Goodman LJ, Trenholme GM, Kaplan RL, Segreti J, Hines D, Petrak R, et al. Empiric antimicrobial therapy of domestically acquired acute diarrhoea in urban adults. Archives of Internal Medicine 1990;150(3):541‐6. [PubMed] [Google Scholar]
Ha 2008 {unpublished data only}
- Ha V. A randomised controlled trial of gatifloxacin versus ciprofloxacin for the treatment of bacillary dysentery in children. http://isrctn.org/ISRCTN55945881 (accessed 14 October 2008). [ISRCTN55945881; Wellcome Trust reference: 061330]
Haltalin 1967 {published data only}
- Haltalin KC, Nelson JD, Ring R, Sladoje M, Hinton LV. Double‐blind treatment study of shigellosis comparing ampicillin, sulphadiazine, and placebo. Journal of Pediatrics 1967;70(6):970‐81. [DOI] [PubMed] [Google Scholar]
Haltalin 1968 {published data only}
- Haltalin KC, Nelson JD, Hinton LV, Kusmiesz HT, Sladoje M. Comparison of orally absorbable and non‐absorbable antibiotics in shigellosis. A double‐blind study with ampicillin and neomycin. The Journal of Pediatrics 1968;72(5):708‐20. [DOI] [PubMed] [Google Scholar]
Haltalin 1968a {published data only}
- Haltalin KC, Nelson JD, Kusmiesz HT, Hinton LV. Comparison of intramuscular and oral ampicillin therapy for shigellosis. Journal of Pediatrics 1968;73(4):617‐22. [DOI] [PubMed] [Google Scholar]
Haltalin 1969 {published data only}
- Haltalin KC, Nelson JD, Kusmies, HT, Hinton LV. Optimal dosage of ampicillin for shigellosis. The Journal of Pediatrics 1969;74(4):626‐31. [DOI] [PubMed] [Google Scholar]
Haltalin 1972 {published data only}
- Haltalin KC, Nelson JD. Failure of furazolidone therapy in shigellosis. American Journal of Diseases of Children 1972;123(1):40‐4. [DOI] [PubMed] [Google Scholar]
Haltalin 1972a {published data only}
- Haltalin KC, Kusmiesz HT, Hinton LV, Nelson JD. Treatment of acute diarrhoea in outpatients. Double‐blind study comparing ampicillin and placebo. American Journal of Diseases of Children 1972;124(4):554‐61. [DOI] [PubMed] [Google Scholar]
Han 1998 {published data only}
- Han CR, Li SL, Luo DD. Comparation of rufloxacin with homefloxacin in 30 cases of bacillary dysentery. Journal of Clinical Internal Medicine 1998;15(2):88. [Google Scholar]
Hansson 1981 {published data only}
- Hansson HB, Barkenius G, Cronberg S, Juhlin I. Controlled comparison of nalidixic acid or lactulose with placebo in shigellosis. Scandinavian Journal of Infectious Diseases 1981;13(3):191‐3. [DOI] [PubMed] [Google Scholar]
Helvaci 1998 {published data only}
- Helvaci M, Bektaslar D, Ozkaya B, Yaprak I, Umurtak B, Ertugrul A. Comparative efficacy of cefixime and ampicillin‐sulbactam in shigellosis in children. Acta Paediatrrica Japonica 1998;40:131‐4. [DOI] [PubMed] [Google Scholar]
Hiraishi 1980 {published data only}
- Hiraishi K, Matsubara Y. Comparison of clinical efficacy of fosfomycin (FOM) and kanamycin (KM) in bacillary dysentery by double blind method. Kansenshogaku Zasshi. Journal of the Japanese Association for Infectious Diseases 1980;54(7):343‐52. [DOI] [PubMed] [Google Scholar]
Imagawa 1988 {published data only}
- Imagawa Y, Tomizawa I, Takizawa Y, Ito K, Matsubara Y, Seo T, et al. Comparison of clinical efficacy of ciprofloxacin (CPFX, BAY o 9867) and pipemidic acid (PPA) in the treatment of infectious enteritis by a double‐blind method. Journal of the Japanese Association for Infectious Diseases 1988;62(4):322‐39. [DOI] [PubMed] [Google Scholar]
Iushchuk 2007 {published data only}
- Iushchuk ND, Maev IV, Mar'ianovskaia TV, Gagarina IV. Using neosmectin in the complex therapy of patients with acute intestinal infections. Experimental & Clinical Gastroenterology 2007;2:126‐30. [PubMed] [Google Scholar]
Jiang 1994 {published data only}
- Jiang SC, Wang XF, Miao JZ. Clinical efficacy of ciprofloxacin and ofloxacin. Chinese Journal of Internal Medicine 1994;33(7):444‐8. [PubMed] [Google Scholar]
Jiang 2000 {published data only}
- Jiang XP, Ye Y. Therapeutic effect of phosphonomycin combined with another antibiotic on shigellosis. Journal of Clinical Internal Medicine 2000;17(1):48. [Google Scholar]
Jinhua 1992 {published data only}
- Jinhua L, Ziaofeng W, Suchum J. Effect observation of pefloxacin in treatment of 64 patients with acute bacillary dysentery. Chinese Journal of Infectious Diseases 1992;10(Suppl 2):116‐8. [Google Scholar]
Kabir 1984 {published data only}
- Kabir I, Rahaman MM, Ahmed SM, Akhter SQ, Butler T. Comparative efficacies of pivmecillinam and ampicillin in acute shigellosis. Antimicrobial Agents and Chemotherapy 1984;25(5):643‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Legros 2004 {published data only}
- Legros D, Fontaine O. Shigellosis: report of a workshop, held at ICDDR,B: Centre for Health and Population Research, Dhaka, Bangladesh, on 16‐18 February 2004. Journal of Health, Population & Nutrition 2004;22(4):445‐9. [PubMed] [Google Scholar]
Lexomboon 1972 {published data only}
- Lexomboon U, Mansuwan P, Duangmani C, Benjadol P, M'cMinn MT. Clinical evaluation of cotrimoxazole and furazolidone in treatment of shigellosis in children. British Medical Journal 1972;3(5817):23‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lionel 1969 {published data only}
- Lionel ND, Abeyasekera FJ, Samarasinghe HG, Goonewardena CV, Tawil GS. A comparison of a single dose and a five day course of tetracycline therapy in bacillary dysentery. Journal of Tropical Medicine and Hygiene 1969;72(7):170‐2. [PubMed] [Google Scholar]
Lolekha 1988 {published data only}
- Lolekha S, Patanachareon S, Thanangkul B, Vibulbandhitkit S. Norfloxacin versus co‐trimoxazole in the treatment of acute bacterial diarrhoea: a placebo controlled study. Scandinavian Journal of Infectious Diseases. Supplementum 1988;56:35‐45. [PubMed] [Google Scholar]
Lolekha 1991 {published data only}
- Lolekha S, Vibulbandhitkit S, Poonyarit P. Response to antimicrobial therapy for shigellosis in Thailand. Reviews of Infectious Diseases 1991;13(Suppl 4):S342‐6. [DOI] [PubMed] [Google Scholar]
Mabadeje 1974 {published data only}
- Mabadeje AF. A controlled clinical trial of trimethoprim‐sulphamethoxazole in shigella dysentery. Journal of Tropical Medicine and Hygiene 1974;77(3):50‐4. [PubMed] [Google Scholar]
Mahllooji 2004 {published data only}
- Mahllooji KH. A comparative study of cefixime and nalidixic acid, against Shigella in Children Hospital Medical Center of Ali Asghar in Tehran‐Iran during 1999. International Journal of Infectious Diseases 2004;8(Suppl 1):S17. [Google Scholar]
Martin 2000 {published data only}
- Martin JM, Pitetti R, Maffei R, Tritt J, Smail K, Wald ER. Treatment of shigellosis with cefixime: two days vs. five days. Pediatric Infectious Diseases Journal 2000;19:522‐6. [DOI] [PubMed] [Google Scholar]
Matsuoka 1995 {published data only}
- Matsuoka Y, Irimajiri S, Obana M, Tomizawa I, Takizawa Y, Nitta Y, et al. Clinical study on the effects of grepafloxacin on infectious enteritis assessment of the fecal drug level and intestinal bacterial flora in a patient with infectious enteritis. Japanese Journal of Chemotherapy 1995;43(Suppl 1):319‐32. [Google Scholar]
Miles 1977 {published data only}
- Miles RN. Bacillary dysentery in Cyprus. Journal of the Royal Army Medical Corps 1977;12:342‐4. [Google Scholar]
Mol 1987 {published data only}
- Mol P, Mets T, Lagasse R, Vandepitte J, Mutwewingabo A, Butzler JP. Treatment of bacillary dysentery: a comparison between enoxacin and nalidixic acid. Journal of Antimicrobial Chemotherapy 1987;19(5):695‐8. [DOI] [PubMed] [Google Scholar]
Moolasart 1999 {published data only}
- Moolasart P, Eampokalap B, Ratanasrithong M. Comparison of the efficacy of ceftibuten and norfloxacin in the treatment of acute gastrointestinal infection in children. Southeast Asian Journal of Tropical Medicine and Public Health 1999;30(4):764‐9. [PubMed] [Google Scholar]
Morisawa 1970 {published data only}
- Morisawa T. Clinical effects of Diromo on intestinal diseases (studies by double blind test). Japanese Archives of Internal Medicine 1970;17(4):99‐102. [PubMed] [Google Scholar]
Motohiro 1982 {published data only}
- Motohiro T, Sakata Y, Fujimoto T. Evaluation of the effect of pipemidic acid on enteritis. A double blind controlled study compared with piromidic acid. Chemotherapy 1982;30(2):125‐48. [Google Scholar]
Nelson 1975 {published data only}
- Nelson JD, Haltalin KC. Comparative efficacy of cephalexin and ampicillin for shigellosis and other types of acute diarrhoea in infants and children. Antimicrobial Agents and Chemotherapy 1975;7(4):415‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 1976 {published data only}
- Nelson JD, Kusmiesz H, Jackson LH. Comparison of trimethoprim‐sulphamethoxazole and ampicillin therapy for shigellosis in ambulatory patients. Journal of Pediatrics 1976;89(3):491‐3. [DOI] [PubMed] [Google Scholar]
Nikorowitsch 1978 {published data only}
- Nikorowitsch E. Length of stay in the hospital in shigella and salmonella infections treated with furazolidone and without any antimicrobial chemotherapy [Krankenhausverweildauer von Shigellosen und Salmonellosen bei Behandlung mit Furazolidon und ohne antimikrobielle Chemotherapie]. Zeitschrift Für Die Gesamte Innere Medizin Und Ihre Grenzgebiete 1978;33(2):51‐4. [PubMed] [Google Scholar]
Oldfield 1987 {published data only}
- Oldfield EC, Bourgeois AL, Omar AK, Pazzaglia GL. Empirical treatment of Shigella dysentery with trimethoprim: five day course vs. single dose. American Journal of Tropical Medicine and Hygiene 1987;37(3):616‐23. [DOI] [PubMed] [Google Scholar]
Orenstein 1981 {published data only}
- Orenstein WA, Ross L, Overturf GD, Wilkins J, Redfield DR, Underman A. Antibiotic treatment of acute shigellosis: failure of cefamandole compared with trimethoprim‐sulphamethoxazole and ampicillin. American Journal of the Medical Sciences 1981;282(1):27‐33. [DOI] [PubMed] [Google Scholar]
Ostrower 1979 {published data only}
- Ostrower VG. Comparison of cefaclor and ampicillin in the treatment of shigellosis. Postgraduate Medical Journal 1979;55(Suppl):482‐4. [PubMed] [Google Scholar]
Petruccelli 1992 {published data only}
- Petruccelli BP, Murphy GS, Sanchez JL, Walz S, DeFraites R, Gelnett J, et al. Treatment of traveller's diarrhoea with ciprofloxacin and loperamide. Journal of Infectious Diseases 1992;165(3):557‐60. [DOI] [PubMed] [Google Scholar]
Pichler 1986 {published data only}
- Pichler H, Diridl G, Wolf D. Ciprofloxacin in the treatment of acute bacterial diarrhoea: a double blind study. European Journal of Clinical Microbiology 1986;5(2):241‐3. [DOI] [PubMed] [Google Scholar]
Pichler 1987 {published data only}
- Pichler HE, Diridl G, Stickler K, Wolf D. Clinical efficacy of ciprofloxacin compared with placebo in bacterial diarrhoea. American Journal of Medicine 1987;82(4A):329‐32. [PubMed] [Google Scholar]
Prado 1981 {published data only}
- Prado V, Cohen J, Harun A, Aguirre X, Diaz C. Comparative randomised study on the clinico‐bacteriological effectiveness of mecillinam versus cotrimoxazole in shigellosis. Revista Chilena de Pediatría 1981;52(2):118‐24. [PubMed] [Google Scholar]
Prado 1992 {published data only}
- Prado D, Lopez E, Liu H, Devoto S, Woloj M, Contrini M, et al. Ceftibuten and trimethoprim‐sulphamethoxazole for treatment of Shigella and enteroinvasive Escherichia coli disease. Pediatric Infectious Disease Journal 1992;11(8):644‐7. [PubMed] [Google Scholar]
Rabbani 1982 {published data only}
- Rabbani GH, Gilman RH, Spira WM. Single dose ampicillin therapy for the treatment of Shigellosis in Bangladesh. Southeast Asian Journal of Tropical Medicine and Public Health 1982;13(3):505‐6. [Google Scholar]
Rakhmanova 1996 {published data only}
- Rakhmanova AG, Kulikov VP. Treatment of Flexner's dysentery with ofloxacin [Lechenie dizenterii Fleksnera ofloksatsinom]. Antibiotiki i Khimioterapii 1996;41(9):95. [PubMed] [Google Scholar]
Raqib 2008 {unpublished data only}
- Raqib R. Therapeutic induction of endogenous antibiotics for improved recovery in shigellosis. http://clinicaltrials.gov/show/NCT00800930 (accessed 9 December 2008).
Rogerie 1986 {published data only}
- Rogerie F, Ott D, Vandepitte J, Verbist L, Lemmens P, Habiyaremye I. Comparison of norfloxacin and nalidixic acid for treatment of dysentery caused by Shigella dysenteriae type 1 in adults. Antimicrobial Agents and Chemotherapy 1986;29(5):883‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagara 1993 {published data only}
- Sagara H, Tomizawa I, Takizawa Y, Yamaguchi T, Masuda G, Negishi I, et al. Clinical study of temafloxacin on infectious enteritis. Chemotherapy 1993;41(Suppl. 5):464‐78. [Google Scholar]
Sagara 1994 {published data only}
- Sagara H, Tomizawa I, Takizawa Y, Nitta Y, Tsunoda T, Yamaguchi T, et al. Basic and clinical studies of fleroxacin on infectious enteritis. Research Group of AM‐833 on infectious enteritis. Kansenshogaku Zasshi. Journal of the Japanese Association for Infectious Diseases 1994;68(11):1390‐408. [DOI] [PubMed] [Google Scholar]
Saito 1983 {published data only}
- Saito M, Tomizawa I, Konishi K, Takizawa Y, Matsubara Y, Seo T, et al. Comparison of clinical efficacy of pipemidic acid (PPA) and kanamycin (KM) in bacillary dysentery by double blind method. Kansenshogaku Zasshi 1983;57(4):303‐17. [DOI] [PubMed] [Google Scholar]
Saito 1984 {published data only}
- Saito M, Seo T, Matsubara Y, Tomizawa I, Takizawa Y, Ito K, et al. Comparison of clinical efficacy of ofloxacin COFLX: DL‐8280) and pipemidic acid (PPA) in acute infectious diarrhoea by a double‐blind method. Kansenshogaku Zasshi. Journal of the Japanese Association for Infectious Diseases 1984;58(10):965‐81. [DOI] [PubMed] [Google Scholar]
Salam 1995 {published data only}
- Salam MA, Seas C, Khan WA, Bennish ML. Treatment of shigellosis: IV. Cefixime is ineffective in shigellosis in adults. Annals of Internal Medicine 1995;123(7):505‐8. [DOI] [PubMed] [Google Scholar]
Salam 1999 {published data only}
- Salam MA, Khan WA, Dhar U, Ronan A, Rollins NC, Bennish ML, et al. Vitamin A for treating shigellosis. BMJ 1999;318(7188):939‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sepp 1995 {published data only}
- Sepp E, Tamm E, Torm S, Lutsar I, Mikelsaar M, Salminen S. Impact of a Lactobacillus probiotic on the faecal microflora in children with Shigellosis. Microecology and Therapy 1995;23:74‐80. [Google Scholar]
Seto 1992 {published data only}
- Seto WH, Lau FL, Gotuzzo EH, Carillo C. Lomefloxacin versus trimethoprim/sulphamethoxazole in the treatment of adults with acute bacterial diarrhoea. International Journal of Antimicrobial Agents 1992;2(1):61‐6. [DOI] [PubMed] [Google Scholar]
Soares 1994 {published data only}
- Soares JL, Arendt V, Coue JC, Milleliri JM, Philips B, Regis R, et al. Short‐term ciprofloxacin treatment of bacillary dysentery due to Shigella dysenteriae type 1 in Rwandan refugees [Traitement court par la ciprofloxacine de la dysenterie bacillaire à Shigella dysenteriae type 1 chez des réfugiés Ruandais]. Médecine Tropicale: Revue Du Corps De Santé Colonial 1994;54(4):319‐23. [PubMed] [Google Scholar]
Soares 1996 {published data only}
- Soares JL, Milleliri JM, Pigny N, Dupoux J, Coue JC. Efficacy of bacillare dysentery's treatment by lomefloxacine amongst Rwandese refugees in North Zaire [Efficacité du traitement de la dysenterie bacillaire par lomefloxacine chez des refugies au Nord‐Zaire]. Medecine et Maladies Infectieuses 1996;26(2):141‐4. [Google Scholar]
Study Group 2002 {published data only}
- Zimbabwe, Bangladesh, South Africa (Zimbasa) Dysentery Study Group. Multicenter, randomised, double blind clinical trial of short course versus standard course oral ciprofloxacin for Shigella dysenteriae type 1 dysentery in children. Pediatric Infectious Disease Journal 2002;21(12):1136‐41. [DOI] [PubMed] [Google Scholar]
Tian 1986 {published data only}
- Tian HY, Zhang XM, Zhou JQ. Effectiveness of swertia davidi and TMP in treating 75 cases of acute bacillary dysentery. Chinese Journal of Integrated Traditional and Western Medicine 1986;6(1):34‐5. [PubMed] [Google Scholar]
Tong 1970 {published data only}
- Tong MJ, Martin DG, Cunningham JJ, Gunning JJ. Clinical and bacteriological evaluation of antibiotic treatment in shigellosis. JAMA 1970;214(10):1841‐4. [PubMed] [Google Scholar]
Varsano 1991 {published data only}
- Varsano I, Eidlitz‐Marcus T, Nussinovitch M, Elian I. Comparative efficacy of ceftriaxone and ampicillin for treatment of severe shigellosis in children. Journal of Pediatrics 1991;118(4 (Pt 1)):627‐32. [DOI] [PubMed] [Google Scholar]
Vinh 2000 {published data only}
- Vinh H, Wain J, Chinh MT, Tam CT, Trang PT, Nga D, et al. Treatment of bacillary dysentery in Vietnamese children: two doses of ofloxacin versus 5‐days nalidixic acid. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(3):323‐6. [DOI] [PubMed] [Google Scholar]
Wistrom 1992 {published data only}
- Wistrom J, Jertborn M, Ekwall E, Norlin K, Soderquist B, Stromberg A, et al. Swedish study Group. Empiric treatment of acute diarrhoeal disease with norfloxacin. A randomised, placebo‐controlled study.. Annals of Internal Medicine 1992;117(3):202‐8. [DOI] [PubMed] [Google Scholar]
Xiouying 1986 {published data only}
- Xiouying WU, Juanfeng LU, Ruiyun YE. Therapeutic effect of pipemidic acid in the treatment of bacillary dysentery in children. Chinese Journal of Infectious Diseases 1986;4(Suppl 4):736‐7. [Google Scholar]
Yamamoto 1973 {published data only}
- Yamamoto T, Tsunoda O, Sugiyama S, Nakajima K, Akao M. Effects of lividomycin on bacillary dysentery and related disorders. Comparison with the effects of kanamycin by a double blind method. Kansenshogaku Zasshi. Journal of the Japanese Association for Infectious Diseases 1973;47(2):35‐43. [DOI] [PubMed] [Google Scholar]
Ye 1990 {published data only}
- Ye XL, Cao YX, Xu JS, Wu KY, Shi XY. Multi‐drug‐resistant Shigella infections in Fujian Province, China. Journal of Diarrhoeal Diseases Research 1990;8(3):99. [PubMed] [Google Scholar]
Yin 1998 {published data only}
- Lu Y, Cheng GX. A clinical investigation of treatment of acute bacillary dysentery with ceftriaxone in 84 children. New Chinese Medicine 1998;29(3):130‐1. [Google Scholar]
Yunus 1982 {published data only}
- Yunus M, Rahman ASMM, Farooque AS, Glass RI. Clinical trial of ampicillin v. trimethoprim sulphamethoxazole in the treatment of Shigella dysentery. Journal of Tropical Medicine and Hygiene 1982;85(5):195‐9. [PubMed] [Google Scholar]
Yuying 1995 {published data only}
- Yuying M, Guirong L, Guiqing D, Shuying Y. Clinical efficacy of ciprofloxacin in the treatment of 29 cases acute bacterial dysentery. Chinese Journal of Antibiotics 1995;20(2):110‐1. [Google Scholar]
Zhang 1991 {published data only}
- Zhang YY, Zhun BY, Jiang SC. Clinical evaluation of enoxacin. Chinese Journal of Internal Medicine 1991;30(8):480‐3, 521. [PubMed] [Google Scholar]
References to studies awaiting assessment
Carbo 1981 {published data only}
- Carbo L. Report of a clinical study of the therapeutic agent Ro/12/2510 vs. ampicillin in acute bacillary dysentery by Shigella [Reporte del estudio clinico del agente terapeutico Ro‐12‐2510 vs. ampicilina en cuadros de disenteria bacilar aguda por Shigella]. Investigación Médica Internacional 1981;8(2):191‐3. [Google Scholar]
Additional references
Baer 1999
- Baer JT, Vugia DJ, Reingold AL, Aragon T, Angulo FJ, Bradford WZ. HIV infection as a risk factor for shigellosis. Emerging Infectious Diseases 1999;5(6):820‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2007
- British National Formulary (BNF 53). www.bnf.org/bnf March 2007 (accessed 13 June 2007).
Bogaerts 1983
- Bogaerts J, Verhaegen J, Munyabikali JP, Mukantabana B, Lemmens P, Vandeven J, et al. Antimicrobial resistance and serotypes of Shigella isolates in Kigali, Rwanda (1983 to 1993): increasing frequency of multiple resistance. Diagnostic Microbiology and Infectious Disease 1997;28(4):165‐71. [DOI] [PubMed] [Google Scholar]
CDC 2005
- Center for Disease Control and Prevention, Division of Bacterial and Mycotic Diseases. Shigellosis. www.cdc.gov/node.do/id/0900f3ec8000755c 13 October 2005 (accessed 13 June 2007).
CONSORT 2008
- The CONSORT Statement. Available at http://www.consort‐statement.org (accessed 15 November 2008).
Datta 2003
- Datta D, Bhattacharya MK, Dutta S, Daatta A, Sarkar D, Bhandra B. Emergence of multidrug resistant Shigella dysenteriae type 1 causing sporadic outbreak in and around Kolkata, India. Journal of Health, Population, and Nutrition 2003;21(1):79‐80. [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Higgins, JPT, Altman DG, editors. Analysing and presenting results. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]; Section 8. www.cochrane.org/resources/handbook/hbook.htm (accessed 31 May 2005).
Dutta 2002
- Dutta S, Rajendran K, Roy S, Chatterjee A, DuttaP, Nair GB, et al. Shifting serotypes, plasmid profile analysis and antimicrobial resistance pattern of shigellae strains isolated from Kolkata, India during 1995‐2000. Epidemiology and infection 2002;129(2):235‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2004 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2004.
Gupta 2004
- Gupta A, Polyak CS, Bishop RD, Sobel J, Mintz ED. Laboratory‐confirmed shigellosis in the United States, 1989‐2002: epidemiologic trends and patterns. Clinical Infectious Diseases 2004;38(10):1372‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Huebner 1993
- Huebner J, Czerwenka W, Gruner E, Graevenitz A. Shigellemia in AIDS patients: case report and review of the literature. Infection 1993;21(2):122‐4. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosek 2008
- Kosek M, Yori PP, Pan WK, Olortegui MP, Gilman RH, Perez J, et al. Epidemiology of highly endemic multiply antibiotic‐resistant shigellosis in children in the Peruvian Amazon. Pediatrics 2008;122:e541‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotloff 1999
- Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization 1999;77(8):651‐66. [PMC free article] [PubMed] [Google Scholar]
Kuo 2008
- Kuo CY, Su LH, Perera J, Carlos C, Tan BH, Kumarasinghe G, et al. Antimicrobial susceptibility of Shigella isolates in eight Asian countries, 2001‐2004. Journal of Microbiology, Immunology and Infection 2008;41:107‐11. [PubMed] [Google Scholar]
Mates 2000
- Mates A, Eyny D, Philo S. Antimicrobial resistance trends in Shigella serogroups isolated in Israel, 1990 ‐ 1995. European Journal of Clinical Microbiology & Infectious Diseases 2000;19(2):108‐11. [DOI] [PubMed] [Google Scholar]
Niyogi 2005
- Niyogi SK. Shigellosis. Journal of Microbiology 2005;43(2):133‐43. [PubMed] [Google Scholar]
Park 2005
- Park K. Park's textbook of preventive and social medicine. 18th Edition. Jabalpur: M/S Banarsidas Bhanot Publishers, 2005. [Google Scholar]
Pazhani 2004
- Pazhani GP, Sarkar B, Ramamurthy T, Bhattacharya SK, Takeda Y, et al. Clonal multidrug‐resistant Shigella dysenteriae type 1 strains associated with epidemic and sporadic dysenteries in eastern India. Antimicrobial Agents and Chemotherapy 2004;48(2):681‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pazhani 2008
- Pazhani GP, Niyogi SK, Singh AK, Sen B, Taneja N, Kundu M, et al. Molecular characterization of multidrug‐resistant Shigella species isolated from epidemic and endemic cases of shigellosis in India. Journal of Medical Microbiology 2008;57:856‐63. [DOI] [PubMed] [Google Scholar]
Review Manager 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sarkar 2003
- Sarkar K, Ghosh S, Nujogi SK, Bhattacharya SK. Shigella dysenteriae type 1 with reduced susceptibility to flouroquinolones. Lancet 2003;361(9359):785. [DOI] [PubMed] [Google Scholar]
Shane 2003
- Shane AL, Tucker NA, Crump JA, Mintz ED, Painter JA. Sharing Shigella: risk factors and costs of a multi‐community outbreak of shigellosis. Archives of Pediatric and Adolescent Medicine 2003;157(6):601‐3. [DOI] [PubMed] [Google Scholar]
Shiferaw 2004
- Shiferaw B, Shallow S, Marcus R, Segler S, Soderlund D, Hardnett FP, et al. Emerging Infections Program FoodNet Working Group. Trends in population‐based active surveillance for shigellosis and demographic variability in FoodNet sites, 1996‐1999. Clinical Infectious Diseases 2004;38 Suppl 3:175‐80. [DOI] [PubMed] [Google Scholar]
Sinha 1987
- Sinha AK, Bhattacharya SK, Sen D, Sengupta PG, Pal SC. Leukemoid reaction in Shigella dysenteriae type 1 infections. Indian Journal of Medical Research 1987;85:500‐2. [PubMed] [Google Scholar]
Sire 2008
- Sire JM, Macondo EA, Clude JDPG, Siby T, Bahsoun I, Seck A, et al. Antimicrobial resistance in Shigella species isolated in Dakar, Senegal (2004‐2006). Japanese Journal of Infectious Disease 2008;61:307‐9. [PubMed] [Google Scholar]
StatCalc 2006 [Computer program]
- Krishnamurthy K. StatCalc 2.0. University of Louisiana at Lafayette, 2006.
Sur 2003
- Sur D, Niyogi SK, Sur S, Datta KK, Takeda Y, Nair GB, et al. Multidrug resistant Shigella dysenteriae type 1: forerunners of a new epidemic strain in eastern India?. Journal of Emerging Infectious Diseases 2003;9(3):404‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sur 2004
- Sur D, Ramamurthy T, Deen J, Bhattacharya SK. Shigellosis: challenges and management issues. Indian Journal of Medical Research 2004;120(5):454‐62. [PubMed] [Google Scholar]
Talukder 2004
- Talukder KA, Khajanchi BK, Islam MA, Dutta DK, Islam Z, et al. Genetic relatedness of ciprofloxacin‐resistant Shigella dysenteriae type 1 strains isolated in south Asia. Journal of Antimicrobial Chemotherapy 2004;54(4):730‐4. [DOI] [PubMed] [Google Scholar]
Thorpe 2001
- Thorpe CM, Smith WE, Hurley BP, Acheson DW. Shiga toxins induce, superinduce, and stabilize a variety of C‐X‐C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infections and Immunity 2001;69:6140‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Seidlein 2006
- Seidlein L, Kim DR, Ali M, Lee H, Wang X, Thiem VD, et al. A multi centre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Medicine 2006;3(9):e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2005a
- World Health Organization. Dept. of Child and Adolescent Health and Development. Guidelines for the control of Shigellosis, including epidemics due to Shigella dysenteriae type 1. Geneva: World Health Organization, 2005. [Google Scholar]
WHO 2005b
- World Health Organization. Shigellosis: disease burden, epidemiology and case management. Weekly Epidemiological Record 2005;80(11):94‐9. [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Shigella: Disease burden. www.who.int/vaccine_research/diseases/shigella/en (accessed 15 September 2006).


