Despite the large differences in the polysaccharide constituents of the cell walls of eudicots and grasses, the glycome profiles are common to Golgi membranes from both Arabidopsis and maize.
Abstract
The plant endoplasmic reticulum-Golgi apparatus is the site of synthesis, assembly, and trafficking of all noncellulosic polysaccharides, proteoglycans, and proteins destined for the cell wall. As grass species make cell walls distinct from those of dicots and noncommelinid monocots, it has been assumed that the differences in cell-wall composition stem from differences in biosynthetic capacities of their respective Golgi. However, immunosorbence-based screens and carbohydrate linkage analysis of polysaccharides in Golgi membranes, enriched by flotation centrifugation from etiolated coleoptiles of maize (Zea mays) and leaves of Arabidopsis (Arabidopsis thaliana), showed that arabinogalactan-proteins and arabinans represent substantial portions of the Golgi-resident polysaccharides not typically found in high abundance in cell walls of either species. Further, hemicelluloses accumulated in Golgi at levels that contrasted with those found in their respective cell walls, with xyloglucans enriched in maize Golgi, and xylans enriched in Arabidopsis. Consistent with this finding, maize Golgi membranes isolated by flotation centrifugation and enriched further by free-flow electrophoresis, yielded >200 proteins known to function in the biosynthesis and metabolism of cell-wall polysaccharides common to all angiosperms, and not just those specific to cell-wall type. We propose that the distinctive compositions of grass primary cell walls compared with other angiosperms result from differential gating or metabolism of secreted polysaccharides post-Golgi by an as-yet unknown mechanism, and not necessarily by differential expression of genes encoding specific synthase complexes.
INTRODUCTION
The primary walls of plants are assembled from several kinds of polysaccharides, structural proteins, and phenylpropanoids. Cellulose microfibrils can hydrogen-bond with four kinds of hemicellulosic polysaccharides: xyloglucans (XyGs), (glucuronoarabino)xylans (GAX), (gluco)mannans, and (1→3),(1→4)-β-d-glucans (mixed-linkage β-glucans; Scheller and Ulvskov, 2010). Angiosperm XyGs are (1→4)-β-d-glucan backbones branched at the O-6 by Xyl residues that, in some species, can be further substituted by Gal and Fuc residues. (Gluco)mannans are also (1→4)-β-d-linked backbones with interspersed stretches of glucan and mannan, with occasional branching of the mannan moieties at the O-6 position with Gal residues. The GAX polysaccharides are (1→4)-β-d-xylan backbones bearing side-groups of GlcA, 4-O-Me-GlcA, and Ara residues. The microfibrillar scaffold of cellulose and hemicelluloses is embedded in a matrix of two kinds of pectic polysaccharide backbones of (1→4)-α-d-homogalacturonan (HG) and repeating units of the O-2-α-l-Rha-(1→4)-α-d-Gal disaccharide in rhamnogalacturonan-I (RG-I; Caffall and Mohnen, 2009). The HGs can be branched with Xyl residues to form Xyl-HGs, or with four complex oligosaccharides to form RG-II, a polysaccharide that forms dimers with boron di-diester crosslinks. The Rha residues of RG-I can be substituted with branched (1→5)-α-l-arabinans and type-I (1→4)-α-d-galactans with appendant Ara residues. Hydroxyproline- and glycine-rich structural proteins interact with the matrix polysaccharides in plant cell walls, and glycophosphatidylinositol (GPI)-anchored peptidoglycans, such as type-II arabinogalactan-proteins (AGPs), with their highly branched (1→3),(1→6)-,(1→3),(1→6)-β-d-galactan chains and Ara side-groups, are also found in primary cell walls (Showalter, 1993). In addition to structural proteins, the cell wall also contains hundreds of remodeling enzymes, such as expansins and transglucosylases, polysaccharide hydrolases and lyases, oxido-reductases, and proteases (Boudart et al., 2005; Hervé et al., 2016).
Primary cell walls of angiosperm species are classified into two main types, each of distinctive composition (Carpita and Gibeaut, 1993). XyGs are the major hemicellulose in the Type-I walls of dicots and noncommelinid monocots, and contain a matrix of HG and RG-I pectic polysaccharides (Caffall and Mohnen, 2009; Scheller and Ulvskov, 2010). By contrast, Type-II walls of grasses and other commelinid monocots have very little pectin, and contain mostly hemicellulosic GAX (Carpita and Gibeaut, 1993). The mixed-linkage β-glucan unique to the Poales is found at certain stages of primary wall formation. Small amounts of XyG are also found but with side-chains terminated by Gal, thus lacking the terminal Fuc typically found in dicot XyGs (Carpita et al., 2001). Type-I walls incorporate predominantly Hyp- and Gly-rich structural proteins at the end of growth, whereas phenylpropanoids are deposited in the Type-II wall (Carpita and Gibeaut, 1993).
Because flowering plants have two distinct types of primary cell walls, it might be expected that dicots and grasses possess Golgi with synthase complexes reflecting the specific kinds of wall polymers that accumulate. The pectic and noncellulosic polysaccharide constituents of plant cell walls are made within the endoplasmic reticulum (ER)-Golgi apparatus and exported to the cell surface (Nebenführ and Staehelin, 2001). Numerous structural and enzymatic glycoproteins that function at the plasma membrane (PM) and in the cell wall also transit through the Golgi. Cellulose and callose synthases are trafficked through the Golgi apparatus, as are receptor-like kinases, AGPs, other GPI-anchored proteins, and components of the cytoskeletal PM cell–wall signaling matrix (Dunkley et al., 2006; Heazlewood et al., 2007; Nikolovski et al., 2012). Although we have a reasonably complete inventory of the polysaccharides and proteins that are components of cell walls, we have only a modest knowledge of enzymes that synthesize these wall components in the Golgi and how elements of the trafficking machinery chaperone their delivery to the cell surface (Parsons et al., 2012, 2013; Rosquete et al., 2018). To test the hypothesis that cell-wall composition reflects the biosynthetic capacity of the Golgi, we undertook comparative proteome and glycome studies of intact Golgi isolated from etiolated coleoptiles of the commelinid grass model maize (Zea mays) to compare with those from developing leaves of the eudicot model Arabidopsis (Arabidopsis thaliana), as species representative of these two distinct types of primary cell walls.
Equilibrium Suc density-gradient centrifugation has been used to enrich Golgi membranes for proteomics analyses, with relative protein and marker abundance across fractions used to infer the Golgi-specific complement within the mixed membrane preparation (Dunkley et al., 2004, 2006; Nikolovski et al., 2012). Because of contamination by other membranes in conventional downward centrifugation, flotation centrifugation in Suc gradients substantially improves purity of Golgi membranes (Balch et al., 1984; Lending et al., 1990). Its rapid enrichment of Golgi membranes preserves protein integrity required for in vitro polysaccharide synthase reactions and studies of synthase topology (Gibeaut and Carpita, 1993; Urbanowicz et al., 2004). We show here that flotation centrifugation provides a convenient and abundant source of ER and Golgi membranes for comparative analysis of their associated proteins and carbohydrates.
Contrary to expectations, an enzyme-linked immunosorbent assay (ELISA)-based screen using monoclonal antibodies (mAbs) directed against noncellulosic plant cell-wall glycan epitopes and complementary quantitative linkage analysis showed that Golgi from both Arabidopsis and maize have the capacity to make a broad range of cell-wall polysaccharides and are not specifically enriched in those associated with either wall type. Epitopes associated with arabinoxylans were found in Type-II maize walls, but the vast majority of the epitopes recognized in Golgi membranes were in nonfucosylated XyG (Non-Fuc XyG) and RG-I/arabinogalactan (AG) classes more common to Type-I walls of Arabidopsis, including epitopes scarcely represented in the maize wall. In contrast with their abundances in the cell wall, Golgi of Arabidopsis were enriched in xylan epitopes, with few XyG epitopes. Quantitative monosaccharide and linkage analyses confirmed the relative abundance of polysaccharides inferred from the glycome arrays for both Golgi and cell walls.
An alternative means of enrichment of Golgi membranes for proteomics analysis is free-flow electrophoresis (FFE), where membranes are resolved from other organelles and microsomal membranes by differences in surface charges in a continuous liquid stream (Parsons et al., 2014). Preliminary enrichment from crude membranes by alternative means improves protein resolution using this technique. For example, isolation of PMs by two-phase aqueous partitioning enhances the quality of subsequent separations by FFE for proteome analysis (de Michele et al., 2016). Consistent with this enrichment strategy, FFE of Golgi membranes isolated by flotation centrifugation showed a substantial enhancement of luminal and intrinsic proteins of Golgi membranes and associated ER from more loosely associated extrinsic proteins and protein contaminants. We identified >200 maize Golgi proteins known to function in the biosynthesis and metabolism of cell-wall polysaccharides common to all angiosperms, and not just those specific to maize walls. Because the Golgi membranes could be resolved into several subfractions, we showed further that most Golgi-associated proteins were asymmetrically distributed into subdomains dominated by ER-resident proteins, Golgi-resident proteins, and a third domain enriched with cellulose synthase (CesA) proteins destined for trafficking to the PM. These findings prompt a reevaluation of the relative roles of gene expression, polysaccharide synthesis, and trafficking, and the turnover of cell-wall components in transit to and from the PM in determining primary wall composition.
RESULTS
Preparation of Cell Walls and Golgi Membranes
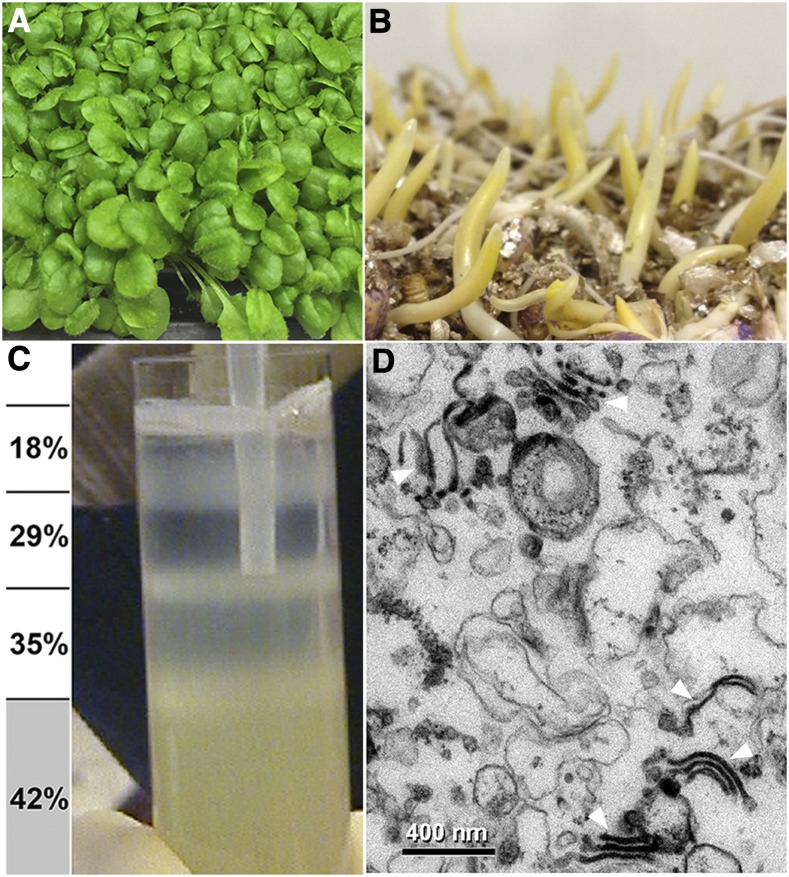
In triplicate experiments, 3-week–old light-grown Arabidopsis seedlings and 2.5- to 3-d–old etiolated maize coleoptiles were gently mashed in an equal volume of 84% (w/v) Suc buffer, and homogenates were squeezed through nylon mesh to separate a cell-wall “mat” from cellular membranes and cytoplasmic material (Supplemental Figure 1). A rich source of intact Golgi membranes was then acquired by flotation centrifugation (Figures 1A to 1C). Numerous Golgi stacks in these preparations could be identified using transmission electron microscopy (TEM; Figure 1D). The Golgi-enriched membranes were then used for glycome array analysis, linkage analysis, and FFE for proteomic analysis. Cell walls purified from the screened mats were sequentially extracted with the chelator ammonium oxalate, a calcium-chelating agent, and increasing concentrations of alkali from 0.1 to 4 M, to yield their respective pectic and hemicellulosic polysaccharides for glycome and linkage analysis (Supplemental Figure 1).
Figure 1.
Isolation of Arabidopsis and Maize Golgi Membranes by Flotation Centrifugation.
(A) Leaves of Arabidopsis seedlings, grown as a dense lawn for 21 ± 1 d, and (B) 2.5- to 3-d–old etiolated maize coleoptiles were overlaid with an equal volume of homogenization buffer with 84% (w/v Suc) and gently mashed in a chilled mortar and pestle. Membranes and organelles in the homogenate were squeezed through nylon mesh, and the homogenate adjusted to between 40% and 45% Suc and placed at the bottom of the centrifuge tube. Step gradients with 35%, 29%, and 18% Suc are overlaid. Tubes were centrifuged in a model no. SW28 Swinging Bucket Rotor (Beckman) at 27,000 rpm (131,500 × g at rmax).
(C) Enriched maize and Arabidopsis Golgi membranes float upwards to the interface of the 35%/29% interface, and ER to the 18%/29% interface. Mitochondria, PM, plastids, and other cellular debris either pellet or remained trapped in the homogenate fraction.
(D) Transmission electron micrograph of fixed and embedded preparations of a maize 29%/35% interface contain numerous Golgi bodies (arrows). Scale bar = 400 nm.
Glycome and Linkage Analyses Confirm Distinctive Arabidopsis Type-I and Maize Type-II Cell-Wall Compositions
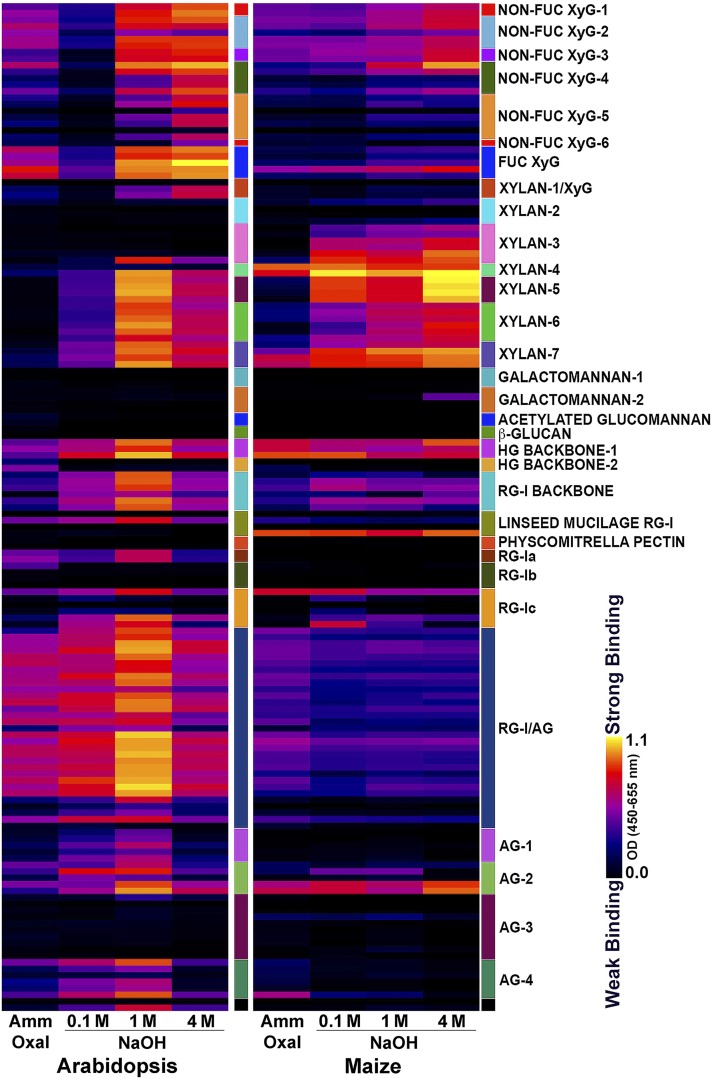
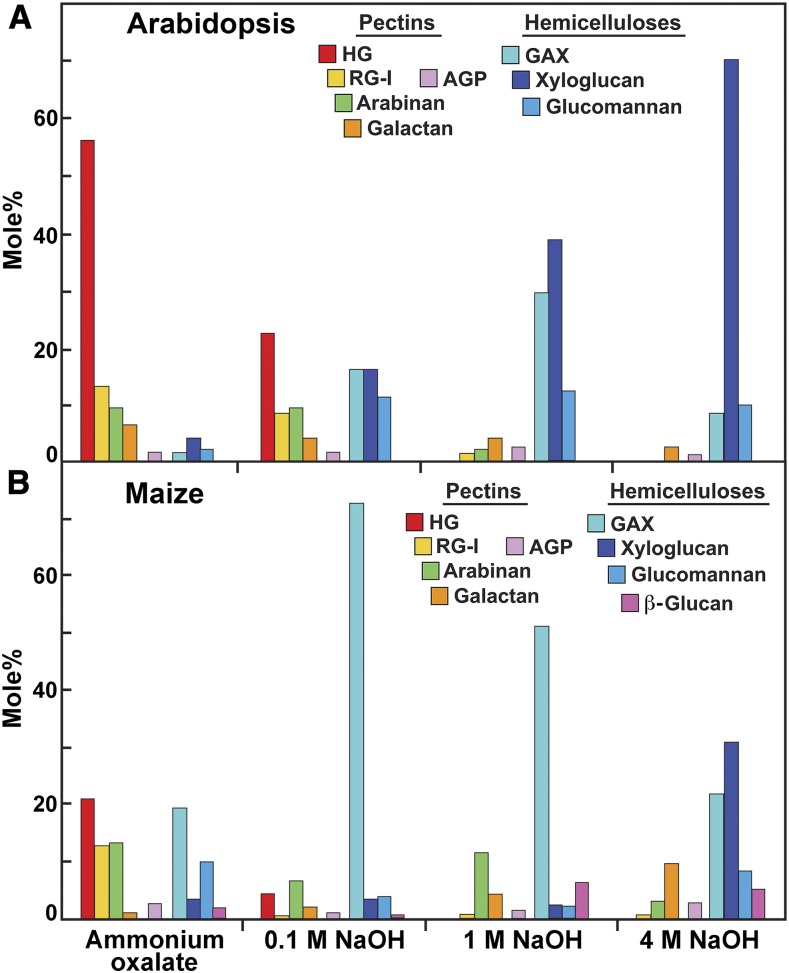
The immunosorbent assay-based glycome analyses utilize libraries of mAbs directed against diverse epitopes present in different kinds of pectins, hemicelluloses, and arabinogalactans of flowering plants (Puhlmann et al., 1994; Steffan et al., 1995; Pattathil et al., 2010, 2015; Schmidt et al., 2015; Dallabernardina et al., 2017; Ruprecht et al., 2017). We used here a library of 155 mAbs that recognize a broad range of noncellulosic carbohydrate epitopes (Supplemental Figure 2; Pattathil et al., 2015). Cell walls from isolated from 3-week–old light-grown seedlings of Arabidopsis and from 2.5 to 3-d–old etiolated maize coleoptiles (and undeveloped leaves within) were sequentially extracted with ammonium oxalate and increasing concentrations of alkali to extract glycan constituents according to the degree and extent of their integration and cross-linking into the walls. The glycome analyses of the Arabidopsis ammonium oxalate and 0.1-M alkali extracts of the cell wall showed epitope abundances consistent with pectic epitopes of HG, RG-I, and associated arabinans and galactans, with significant amounts of XyG epitopes also being extracted by oxalate. The stronger alkali solutions extracted mostly epitopes associated with fucosylated and galactosylated XyGs and xylans, with smaller amounts of pectic epitopes being present in the 4-M NaOH extract (Figure 2). As glycome arrays provide only an indication of the relative proportions of each epitope, we used linkage analysis to quantify the mole percent of component polysaccharides. For linkage analyses, uronic acid residues were carboxyl-reduced with sodium borodeuteride to their respective neutral sugars in extractions of cell walls by ammonium oxalate and 0.1-M NaOH, so that their relative abundance with neutral sugars could be determined by GC-MS. The proportions of pectic and hemicellulosic polysaccharides are inferred from diagnostic linkages of specific polysaccharide backbones, their branch-point residues, and appendant side-groups (Supplemental Table 1). Linkage analysis of equivalent fractions from Arabidopsis cell walls showed that the ammonium oxalate and 0.1 M NaOH extracts were enriched in RG-I and HG, with 4-GalA, and 2- and 2,4-Rha as the most abundant linkages, whereas the 1 M and 4 M alkali extracts of Arabidopsis cell walls were enriched in t-Xyl, 2-Xyl, t-Gal, 2-Gal, and 4 and 4,6-Glc characteristic of fucosylated XyGs (Figure 3A; Supplemental Table 2).
Figure 2.
Glycome Analyses of Chelator- and Alkali-Soluble Fractions of Isolated Cell Walls of Arabidopsis and Maize.
Glycome profiles of fractions obtained by sequential extraction of isolated cell walls, followed by screening of the wall extracts against a collection of plant glycan-directed mAbs. Relative intensities of binding of mAbs are represented as heatmaps, with bright yellow depicting strongest intensity and black depicting undetected. Classes of polysaccharides and glycan epitopes are indicated on the right by small colored boxes. The list of mAbs for each polysaccharide class can be found in Supplemental Figure 2. Lanes represent the relative abundance of polysaccharide epitopes extracted sequentially with hot ammonium oxalate (Amm Oxal), followed by 0.1 M, 1.0 M, and 4.0 M NaOH.
Figure 3.
Polysaccharides and Glycans Represented in Chelator- and Alkali-Soluble Fractions of Isolated Cell Walls From Arabidopsis and Maize.
(A and B) Linkage groups diagnostic for specific polysaccharides were pooled, and nonreducing terminal residues common to more than one polysaccharide were apportioned according to the abundance of their respective branch point residues. Protocols for polysaccharide assignment are described in Supplemental Table 1. Abundances of diagnostic linkages for established polysaccharide structures are in Supplemental Tables 2 and 3.
Glycome analyses of chelator and alkali extracts of isolated cell walls from maize coleoptiles showed epitope abundances consistent with their known pectin, GAX, and XyG constituents. Maize ammonium oxalate and 0.1-M alkali extracts contained small amounts of mostly HG, RG-I, and Non-Fuc XyG epitopes, with abundant GAX epitopes present in the 0.1-M alkali extract. One- and 4-M alkali extracts contained epitopes mainly associated with GAX and smaller amounts of Non-Fuc XyG and HG backbone epitopes (Figure 2). Linkage analyses of maize cell-wall fractions confirmed the presence of 4-GalA, and 2- and 2,4-Rha, consistent with the presence of pectic HG and RG-I polysaccharides in the ammonium oxalate and 0.1-M NaOH extracts, and also showed higher abundances of t-GlcA, t-Ara, and 4- and 3,4-Xyl linkages typical of highly substituted GAX (Figure 3B; Supplemental Table 3). The stronger alkali fractions of maize cell walls were dominated by t-Ara, and 4- and 3,4-Xyl, but also contained t-Xyl, and 4 and 4,6-Glc typical of XyGs, as well as 3- and 4-linked Glc associated with the mixed-linkage β-glucans. In summary, the relative proportions of pectins and hemicelluloses determined by both glycome array and linkage analyses were fully consistent with the compositions characteristic of Type-I and Type-II cell walls.
Glycome and Linkage Analyses Show Contrasts between Carbohydrate Compositions of Arabidopsis and Maize Cell Walls and their Respective Golgi
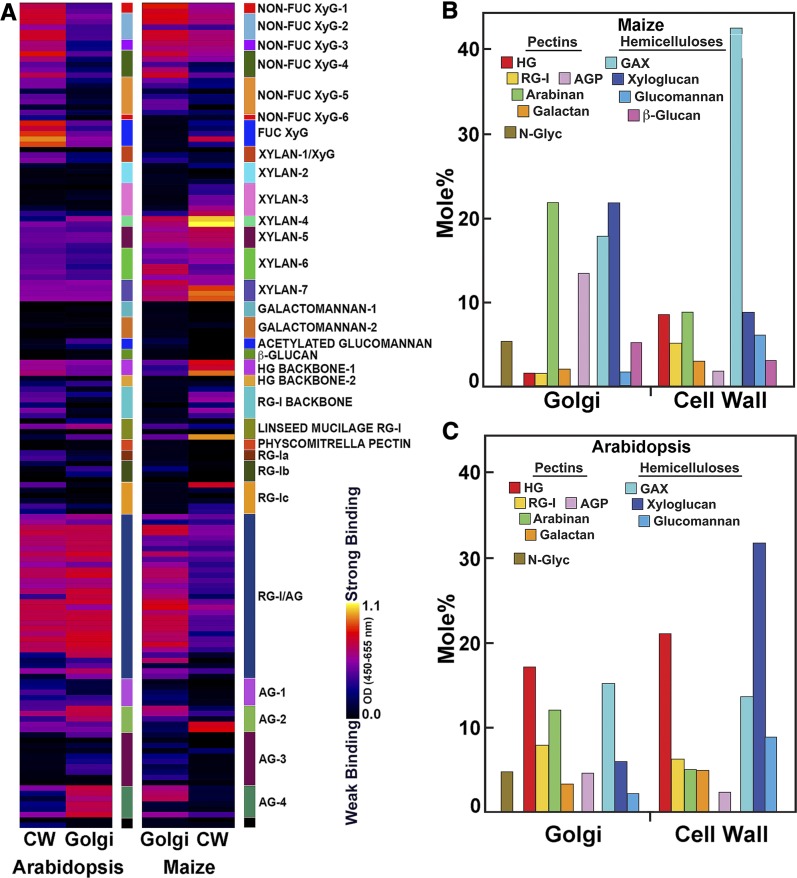
The glycome profiles of the Golgi-resident glycans from both species were different in their epitope compositions from their respective total walls (Figure 4A). Absorbance values for each epitope of noncellulosic polysaccharides extracted from the cell walls by each of the ammonium oxalate and alkali extracts (Figure 2) were scaled by their weight % contribution to the total mass to obtain an estimate of the relative contribution of these noncellulosic epitopes to cell-wall composition. Glycome analyses showed that XyG epitopes (both fucosylated and galactosylated forms) and pectic RG-I dominate the Arabidopsis total wall composition, but xylan and HG epitopes were also detected (Figure 4A). The abundances of arabinan and galactan epitopes recognized by the RG-I/AG mAb clade inferred by binding intensity were relatively lower compared with those of xylan backbones (as recognized by the Xylan-6 and -7 mAbs), GlcA-substituted xylans (CCRC-M150), 4-O-Me-substituted xylans GlcA- (Xylan-5 mAbs) and Ara-substituted (CCRC-M154) xylans, and galactosylated XyGs (non-Fuc XyG-1 CCRC-M95 and CCRC-M101). By contrast, Arabidopsis Golgi membranes were dominated by epitopes recognized by the RG-I/AG, AG-2, and AG-4 mAb clades, with relatively less abundant HG, xylan, and XyG epitopes (Figure 4A). The glycome profiles of maize Golgi contained approximately equivalent binding of mAbs recognizing galactosylated XyGs, side-chain and backbone epitopes of xylans, and arabinogalactan epitopes recognized by the RG-I/AG, AG-2, and AG-4 mAbs. Apart from epitopes of two AG-2 mAbs (CCRC-M133 and CCRC-M107) detected in the walls of maize, arabinogalactan epitopes recognized by AG-4 and other AG-2 mAbs, largely unbranched 6-linked Gal residues (Ruprecht et al., 2017), were abundant in Arabidopsis and maize Golgi but were of relatively low abundance in their walls.
Figure 4.
Comparative Glycome and Polysaccharide Profiles of Cell Wall and Golgi From Maize Etiolated Coleoptiles and Arabidopsis Seedlings.
(A) Glycome profiles of fractions obtained by sequential extractions of isolated maize and Arabidopsis cell walls compared with those from Golgi membranes screened against a collection of plant glycan-directed mAbs. The cell-wall intensities are a composite of relative epitope contributions in chelator- and alkali-soluble fractions (Figure 2). The Golgi relative intensities were normalized to adjust for differences in loading. Relative intensities of binding of mAbs are represented as heatmaps as described in Figure 2.
In (B) maize and (C) Arabidopsis, the relative proportions of Golgi and pectin and hemicellulosic polysaccharides sequentially extracted in chelator- and alkali-soluble cell-wall fractions. Estimations of polysaccharide abundance were calculated as described in Figure 3. Golgi membranes uniquely contained 2-, 6-, and 3,6-Man residues associated with N-linked glycoproteins (N-Glyc). Protocols for polysaccharide assignment are described in Supplemental Table 1. Abundances of diagnostic linkages for established polysaccharide structures are in Supplemental Tables 2 and 3. Estimations of the mole % of each polysaccharide in total noncellulosic cell wall were scaled to their respective mass contributions to the four chelator- and alkali-soluble fractions described in Figure 3.
Arabinogalactan epitopes recognized by the RG-I/AG mAbs were present with approximately equal intensities in both Golgi and cell walls of Arabidopsis, but relatively more abundant in the Golgi of maize than in walls (Figure 4A). Some Non-Fuc XyG-5 mAbs and HG backbone epitopes were also detected in the maize Golgi. The intense signals from the arabinogalactan epitopes were noteworthy, as these epitopes, for the most part, were barely detected in maize cell walls. Amounts of GAX (Xylan-4 and -5 mAb clades) and pectin backbones were relatively less abundant in Golgi compared with their occurrence in maize cell walls. Further, Non-Fuc XyGs were overrepresented in the Golgi, considering their lower abundance in maize cell walls (Figure 4A).
The mole % values of linkage groups diagnostic of total noncellulosic polymers were compiled from the respective amounts in the four extracts (Figures 4B and 4C). However, consistent with the glycome profiles, the monosaccharide and linkage distributions of maize Golgi membranes were in stark contrast to those of the cell wall (Figure 4B). Type-II maize walls were pectin-poor and rich in GAX; linkages diagnostic of GAX and mixed-linkage β-glucans were found in Golgi membranes, which also contained particularly high abundance of 3-, 6-, and 3,6-Gal residues, characteristic of AGPs (Figure 4B; Supplemental Table 1). In contrast with glycome profiles that showed abundant RG-I/AG and some AG classes of epitopes, linkage analysis indicated that RG-Is represent a much smaller proportion of the walls than AGPs. In contrast with their low abundance in the cell walls of maize, 4-Glc and 4,6-Glc residues associated with XyG were high in the maize Golgi membranes (Figure 4B; Supplemental Table 3). Golgi from both Arabidopsis and maize were enriched in 5-arabinans in proportion to walls, whereas the smaller proportions of type-I (arabino)galactan were generally lower in Golgi compared with walls (Figures 4B and 4C). Golgi membranes of both species were enriched in 2-Man, 6-Man, and 3,6-Man—linkages not detected in cell-wall fractions but characteristic of trimmed N-linked core glycosylation.
Arabidopsis Golgi yielded particularly high abundance of 3-, 6-, and 3,6-Gal residues characteristic of AGPs and the 2-Man, 6-Man, and 3,6-Man linkages of N-linked glycoproteins (Figure 4C; Supplemental Table 2). Although linkages associated with fucosylated XyG were detected in the Arabidopsis Golgi (Supplemental Table 2), XyGs were much more abundant in the maize Golgi than in those of Arabidopsis (Figures 4B and 4C; Supplemental Tables 2 and 3). Conversely, linkages associated with xylans were proportionally higher in Arabidopsis Golgi than in their cell wall.
Enrichment of Maize Golgi Membranes by FFE for Proteomic Analysis
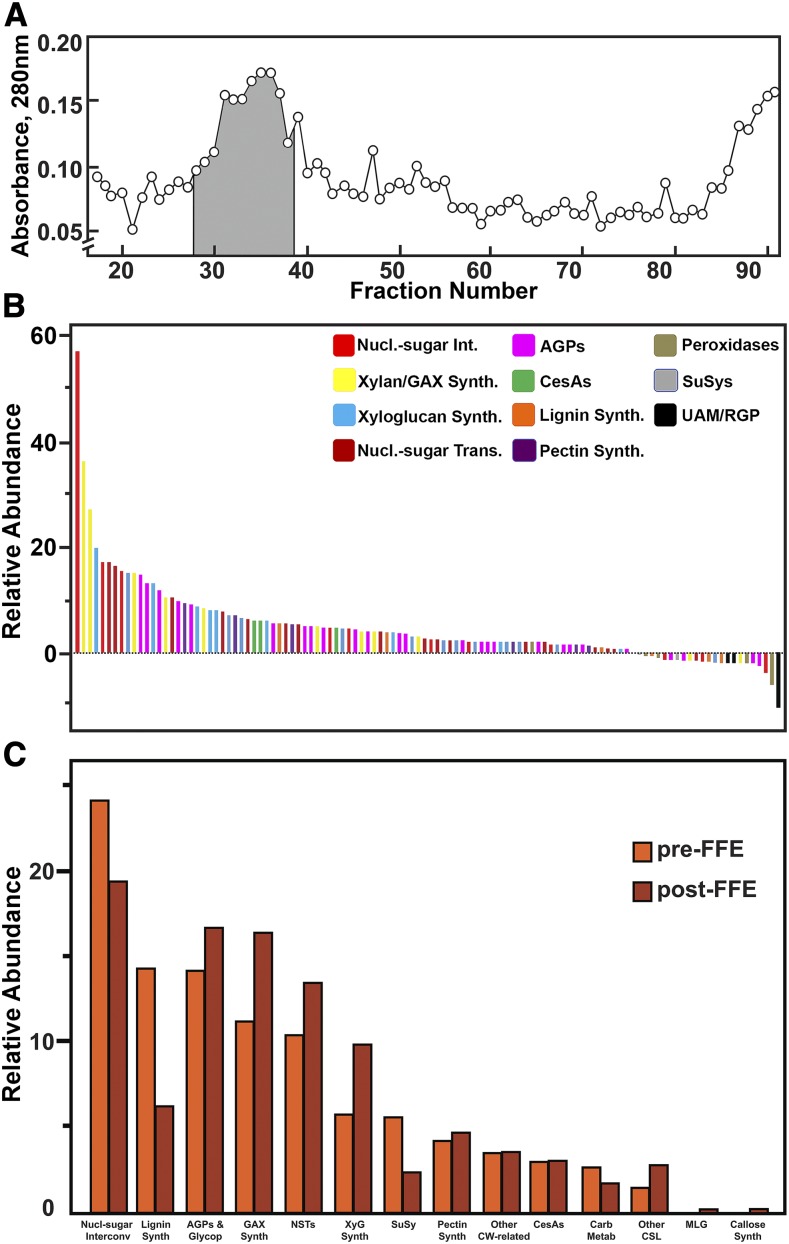
A diverse set of cell-wall–related proteins was identified in Golgi membranes collected after flotation centrifugation (Supplemental Data Set 1). When maize Golgi membranes isolated by flotation centrifugation were enriched further by FFE, the Golgi membranes formed a heterogeneous peak that migrated approximately two-thirds of the distance of the fastest-migrating UV-absorbing material, with only small amounts of other membranes detected in neighboring fractions (Figure 5A). Proteins from triplicate samples after flotation alone (pre-FFE) and those after FFE (post-FFE) were trypsin-digested for subsequent liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Peptide data were processed against maize protein sequence database at Maize Genetics and Genome Database (MaizeGDB; https://www.maizegdb.org), using the software MaxQuant with an integrated Andromeda search engine (Cox and Mann, 2008; Cox et al., 2014) as well as the software Mascot (Matrix Science) for protein identification and relative quantification. Since our original search, we have augmented the public database at MaizeGDB for improved annotation of maize cell-wall protein families (https://www.maizegdb.org/gbrowse/maize_v2test?q=Chr1:1..301354135;label=CellWallGenes). This resource is also posted at http://cellwall.genomics.purdue.edu. The relative abundances of protein distributions across FFE fractions were determined using spectral counts (or MS/MS counts). Spectral counts are the total number of MS/MS spectra of all the nonredundant peptides mapped to a protein or protein family during the entire LC-MS/MS run, and they are referred as MS/MS counts for MaxQuant results. Total MS/MS counts from both MaxQuant and Mascot were normalized to determine the relative enrichment after FFE (Figure 5B). Pairwise comparison of peptide MS/MS counts from MaxQuant among the three independent experiments gave linear regression values of r2 = 0.93 to 0.96 (Supplemental Figure 3A). Identification was considered positive if proteins were found in at least two biological replicates. Identification of a single isoform was considered positive only if it was identified by at least one unique peptide. Identification of protein isoforms is complicated in maize, because many genes share the same sequence as a result of ancestral duplication events. In these instances, the level of expression of similar isoforms was considered as reflective of relative abundance. A total of 2,030 unique proteins were identified at a significance level of P ≤ 0.05 in at least one of 12 Golgi-rich fractions collected after FFE (Supplemental Data Set 2) from combined MaxQuant and Mascot analyses, with 1,168 proteins detected by MaxQuant and 1,781 proteins by Mascot, of which 936 proteins were common to both. Of these FFE-enriched Golgi proteins, ∼11.3% (230) are known to function in cell-wall synthesis and metabolism (Figure 5B; Table 1; Supplemental Figure 4). Application of the maize annotation tool at Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html) gave numerous mis-annotations within their primary descriptions. The protein domain families (PFAM)/Kyoto Encyclopedia of Genes and Genomes (KEGG) descriptions are in general agreement with our maize cell-wall protein database at MaizeGDB, but our manual annotations based on phylogenetic trees constructed with the most similar Arabidopsis and rice (Oryza sativa) sequences refined the specific gene descriptions (Supplemental Data Set 3). Examples of refined annotation include the classes of nucleotide-sugar transporters, CesAs, several glycosyl transferases, and polysaccharide hydrolases. Quantitation of classes of proteins using MapMan functional categories in the Plant Proteomics Database (http://ppdb.tc.cornell.edu/dbsearch/subproteome.aspx; Sun et al., 2009) showed only 2.5% contamination with plastid and mitochondrial proteins (Supplemental Figure 4). PM-associated proteins detected, such as several V-type ATPases and CesAs, traffic through the Golgi, so it is difficult to judge the extent of contamination with PM. Soluble NSF Attachment protein REceptors (SNAREs) and clathrin heavy-chain proteins were also detected that could be Trans-Golgi network (TGN)-related. However, the two maize homologs closest in sequence to an Arabidopsis Synaptophysin61 (SYP61) TGN marker (Groen et al., 2014) were not detected (Supplemental Data Set 4).
Figure 5.
Enrichment of Maize Golgi Proteins by FFE.
(A) Profile of fractions enriched in Golgi proteins. Fractions 27 through 38 (shaded) were collected and pooled into four fractions for proteomics analysis.
(B) Relative enrichment of Golgi proteins was determined by comparison of relative abundance of MS/MS counts from MaxQuant after flotation centrifugation compared with proteins collected in the Golgi fractions after FFE.
(C) Distribution of classes of cell-wall proteins after flotation centrifugation and after FFE.
Table 1. Inventory of protein families identified after free-flow electrophoresis.
| Protein ID | Protein Description Maize GDB (Number of Isoforms) |
|---|---|
| Nucleotide Sugar Interconversion | |
| AUD | UDP-d-Glucuronate Decarboxylase (6) |
| SUD | UDP-d-Glucuronate Decarboxylase (2) |
| GAE | UDP-d-Glucuronate-4-Epimerase (7) |
| UGD | UDP-d-Glu Dehydrogenase (3) |
| UXE | UDP-d-Xyl 4-Epimerase (4) |
| UAM | UDP-l-Ara Mutase (RGP) (6) |
| Nucleotide Sugar Transporters | |
| URGT | UDP-Rha/UDP-Gal Transporter2 (2) |
| UXT | UDP-Xyl Family1 (5) |
| UTR | CMP-Sialic Acid Transporter3 (2) |
| NST-KT1 | Nucleotide Sugar Transporter-KT1 (1) |
| GPT | Glu-6-Phosphate Transporter (2) |
| PPT | Phosphoenolpyruvate Transporter (2) |
| TPT | Triose-Phosphate Transporter (1) |
| GalT | UDP-Gal Transporter (2) |
| UAfT | UDP-Araf Transporter (4) |
| UUAT | UDP-Uronic Acid Transporter (2) |
| Cellulose Synthesis | |
| CesA | Cellulose Synthases (15) |
| CesAL | Cellulose Synthase-Like (CesA-L) (1) |
| Callose Synthesis | |
| GSL | GT48 Glucan Synthase-Like (2) |
| GAX Synthesis | |
| IRX14 | GT43 Xylosyl Transferase (4) |
| IRX9 | GT43 Xylosyl Transferase (3) |
| IRX10L | GT47E Xylan 1,4-β-Xylosyl Transferase (8) |
| GUX1 | GT8A Xylan 1,2-α-GlcA Transferase (1) |
| GT61 | GT61 Glycosyl Transferase (9) |
| Xyloglucan Synthesis | |
| CslC | Xyloglucan β-Glucan Synthase (4) |
| XXT | GT34 Xyloglucan Xylosyl Transferase (10) |
| GT47A | GT47A Galactosyl Transferase (3) |
| FUTL11 | GT37 Xyloglucan FutT11-Like (1) |
| Mixed-Linkage Glucan Synthesis | |
| CslF | Cellulose Synthase-LikeF (4) |
| Other Cellulose Synthase-Like Proteins | |
| CslA | Cellulose Synthase-LikeAs(1) |
| CslD | Cellulose Synthase-LikeD (4) |
| CslE1 | Cellulose Synthase-LikeE (1) |
| Pectin Synthesis | |
| GAUT | GT8D UDP-GalA Transferase (12) |
| AGP and Glycoprotein Synthesis | |
| FLA | Fasciclin-Like Arabinogalactan (3) |
| GT31C | AGP UDP-Gal TransferaseC (3) |
| GT31E | AGP UDP-Gal Transferase (2) |
| HPGT | GT31D Hyp O-Galactosyltransferase (3) |
| GT14 | GT14 β-Glucuronosyltransferase (5) |
| GT16 | GT16 Glycosyl Transferase (1) |
| GT64 | GT64 Glycosyl Transferase (2) |
| GT66 | GT66 Glycosyl Transferase (2) |
| STT3 | N-Oligosaccharyl Transferase Subunit (3) |
| MNS1 | α-Mannosidase (1) |
| RRA | GT77 Extensin UDP-Ara Transferase (3) |
| P4H | Prolyl 4-Hydroxylase (8) |
| FUT | GT10 GDP-3-α-L-Fucosyl Transferase (3) |
| EBS | GT24 BRI1 Suppressor (2) |
| PLT | Phospholipid Transfer Protein (3) |
| Suc Synthase | |
| SuSy | Suc Synthase (5) |
| Monolignol Synthesis | |
| PAL1 | Phe Ammonia-Lyase (8) |
| C3H1 | ρ-Coumarate 3-Hydroxylase (2) |
| C4H | Cinnamate 4-Hydroxylase (3) |
| HCT | ρ-Hydroxycinnamoyl Transferase-Like (1) |
| Polysaccharide Modification | |
| GH9(KOR) | GH9 Glycosyl Hydrolase/KORRIGAN (3) |
| EXPB | β-Expansin (2) |
| XTH | Xyloglucan Endotransglucosylase/Hydrolase (1) |
| BGAL17b | GH35 β-Galactosidase (1) |
| MAN2B1 | Lysosomal α-Mannosidase (1) |
| GluA | Lysosomal β-Glucosidase-Like (1) |
| EH-II | Exohydrolase II (1) |
| GH13 | Glucan 1,3-α-Glucosidase (2) |
| PGaseA | PolygalacturonaseA (1) |
| PGIP1 | Polygalacturonase Inhibitor1 (3) |
| PME | Pectin Methylesterase (1) |
| PL4 | Pectin and Pectate Lyase (1) |
| Other Cell-Wall–Related Proteins | |
| COBRA | COBRA (2) |
| STL | STELLO (GT75-Like) (1) |
| CGL1 | Complex Glycan Less1 (1) |
| CERK | LysM-Chitin Elicitor Receptor Kinase (1) |
| GATL3 | Galacturonosyl Transferase-Like (1) |
| GDPL | Guanosine Diphosphate-Like (1) |
| GT47D | Glycosyl Transferase47 D5 (1) |
| IPUT1 | Inositol Phosphorylceramide GT1 (1) |
| MPL | Cys Protease (2) |
| SKU3 | SKU (GPI-Anchored Cupredoxin) (1) |
Numbers of isoforms identified in each family are in parentheses. A complete list of all cell-wall–related proteins and their MaizeGDB v.3 ID numbers are in Supplemental Data Set 3.
Numerous proteins associated with substrate generation, polysaccharide backbone synthesis, and side-group attachment were identified after FFE, including a more complete collection of the enzymes for nucleotide-sugar interconversion, nucleotide-sugar transport, cellulose and callose synthesis, and mixed-linkage β-glucan synthesis, as well as 25 proteins associated with xylan backbone synthesis and side-group attachment, and 14 proteins associated with AGP synthesis and glycosylation (Table 1; Supplemental Table 4). When total MS/MS counts from MaxQuant analysis were pooled for cell-wall protein classes before and after FFE, relative abundances of glycosyl transferases were generally enriched. By comparison, enzymes of monolignol synthesis, peroxidases, Suc synthases, and certain enzymes of nucleotide-sugar interconversion, particularly the Reversibly Glycosylated Proteins (RGPs), more aptly named Uridine diphosphate (UDP)-arabinopyranose mutases, migrated with Golgi after flotation centrifugation but were depleted in Golgi enriched by FFE (Figures 5B and 5C; Supplemental Table 4).
Comparison of Gene Expression with Maize Cell-Wall Protein Abundance in FFE-Enriched Fractions
Transcripts for 227 of the 230 cell-wall–related proteins identified in the Golgi membranes were detected by RNA sequencing (RNA-Seq) analysis (Figure 6). Certain proteins involved in cellulose synthesis (CesA2, KORRIGAN1c [KOR1c], and KOR1d), four nucleotide sugar interconversion enzymes soluble UDP-d-Glucuronate Decarboxylase3a (SUD3a), SUD3b, UDP-d-glucose dehydrogenase3b (UGD3b), and RGP1b, XyG modification (Xyloglucan Endotransglucosylase/Hydrolase5 [XTH5]), mixed-linkage glucan synthesis (cellulose synthase-likeF2 [CslF2] and CslF4), and lignin synthesis (Phenylalanine Ammonia Lyase2 [PAL2] and Defective Cuticular Ridge family of acetyl transferases [DCR]), had substantially high transcript abundance, but only modest protein abundance. However, two proteins involved in the synthesis of GAX (Glycosyl Transferase61-8 [GT61-8] and GT47 Xylosyl Transferase1g [IRX10-1g]) and one membrane-associated nucleotide sugar dehydrogenase enzyme, membrane-associated UDP-d-Glucuronate Decarboxylase 1c (AUD1c), had high protein abundance with modest to low transcript abundance (Figure 6). Although correlation of transcript level and protein abundance was low across the entire protein set, correlation was much stronger among isoforms encoded by the same family (Supplemental Data Set 4). For this reason, we used transcript abundance to designate the probable isoform abundance for large families of proteins for which protein assignments were ambiguous.
Figure 6.
Comparison of Relative Expression of Cell-Wall–Related Proteins in Etiolated Maize Coleoptiles with Relative Protein Abundance in Golgi Membranes Isolated by FFE.
Abundance of cell-wall–related proteins was derived from MaxQuant MS/MS counts across the 12 fractions of Golgi separated by FFE. Transcripts from RNA-Seq are quantified as numbers of reads per 20-M total. Values for MS/MS counts and transcript abundance were pooled from three independent experiments.
Asymmetric Distribution of Proteins across FFE Fractions
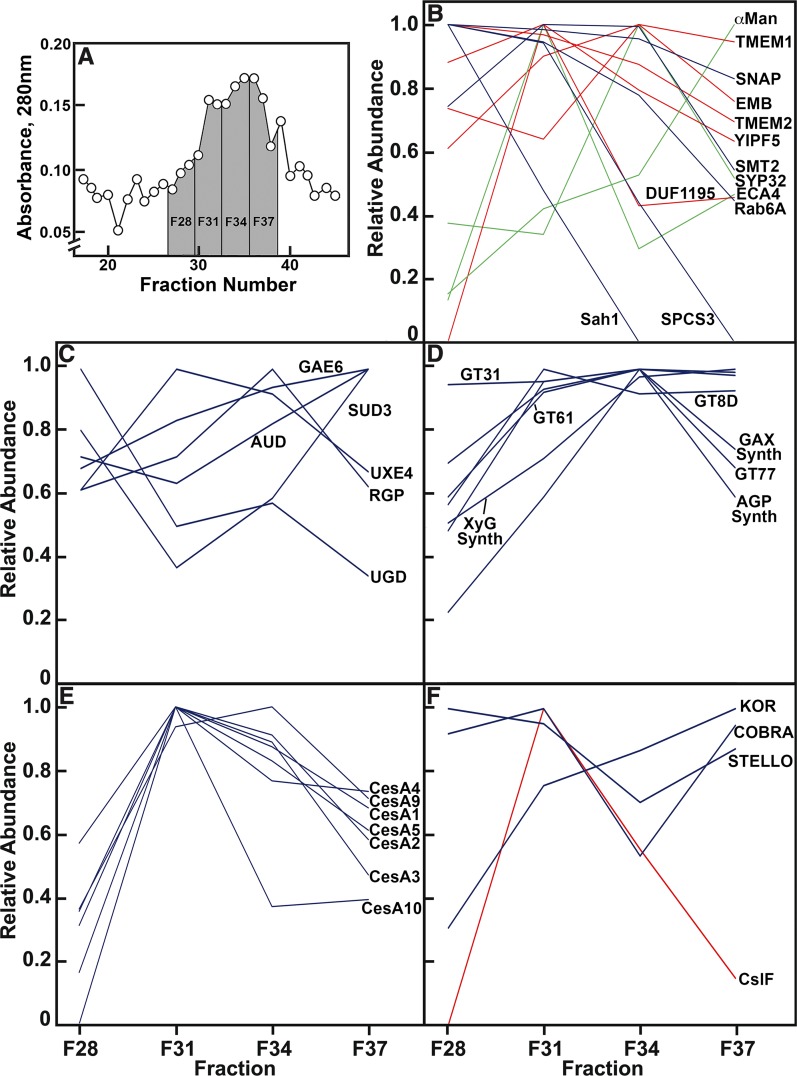
The abundances of proteins confirmed by MaxQuant were strongly correlated with quantitation of spectral counts from Mascot in Golgi membranes separated by FFE over 12 fractions (Supplemental Figure 3B). Once protein identity was established by Mascot and MaxQuant in the Golgi post-FFE, and relative abundance of all cell-wall proteins was well correlated between these two methods (Supplemental Figure 3B), the Mascot spectral counts pooled from all three experiments were used to determine the distribution of each protein across four fractions of pooled Golgi membranes relative to the fraction with the highest spectral counts (Figure 7). Total protein abundance based on absorbance at 280 nm during FFE was maximal in the major peak of Golgi membranes in fractions centered at F34, with a smaller spike of proteins centered at F31, and a small shoulder at F28 (Figure 7A). Numerous established Golgi-localized proteins were enriched in the fractions of the bulk Golgi centered between F34 and F37. These included markers for cis-Golgi, such as a Domain of Unknown Function (DUF616)-containing EMBRYO DEFECTIVE2756, a DUF1195-containing putative sugar transporter, and SYP32, markers for medial-Golgi, such as mannosidase II, and also YIPF5 that has been observed in both the ER and Golgi (Figure 7B; Supplemental Data Set 4). However, some proteins considered to be Golgi-associated had significant representation in the putative ER-rich F28, such as a DUF2359-containing transmembrane protein, TMEM214, and transmembrane protein 115, TMEM115 (Supplemental Data Set 4). ECA4, another Golgi-localized protein, deviated from the bulk Golgi pattern, being enriched in the F31 fraction. The general overlap of these marker proteins across the three Golgi fractions indicated a lack of partitioning of cis-, medial-, and Trans-cisternae. Fraction F28 was ER-enriched based on the distribution of the markers, signal peptidase complex subunit3, sterol methyltransferase2, and S-adenosyl-l-homocysteine hydrolase, although some of these markers migrate to the Golgi fractions to varying extents (Figure 7B). This fraction also contained several 8S, 40S, and 60S ribosomal proteins.
Figure 7.
Relative Abundance of Proteins Across Four Fractions of Golgi Membranes After FFE.
(A) Portion of the profile of fractions from FFE enriched in Golgi proteins.
(B) Profiles of proteins proposed as markers for ER- and Golgi-residence (in blue and red, respectively). Distribution of abundance of three proteins, ECA4, SYP32, and α-Man (in green), did not strictly follow an expected ER- or Golgi-residence profile. SNAP, Synaptosomal nerve-associated protein.
(C) Enzymes of nucleotide-sugar interconversion.
(D) Proteins of pectin and hemicellulose synthesis.
(E) CesAs.
(F) Profiles of three proteins associated with cellulose synthesis, STELLO, KOR, and COBRA (in blue), with CslF proteins (in red).
A complete list of the proteins associated with noncellulosic polysaccharide synthesis and metabolism is provided in Supplemental Data Set 3.
A majority of the enzymes of nucleotide-sugar interconversion were more or less evenly distributed across all fractions, including RGPs, which are extrinsic Golgi proteins not enriched by FFE (Figure 7C). When the abundance of all proteins associated with synthesis of five noncellulosic pectic and hemicellulosic polysaccharides were summed and averaged across each of the four fractions (Supplemental Data Set 4), these values generally aligned with the major Golgi fractions (Figures 7A and 7D). However, a majority of CesA proteins were enriched in F31, a fraction associated with the small spike in absorbance (Figures 7A and 7E). The only other polysaccharide synthase enriched in this fraction was CslF5 (Figure 7F). Other proteins associated with cellulose synthases were not enriched in F31, as STELLO was highly represented in all fractions, and KOR and COBRA were enriched in the bulk Golgi (Figure 7F).
DISCUSSION
The Cell-Wall and Golgi Glycomes of Maize and Arabidopsis
The characteristic differences between Type-I and Type-II cell walls (Carpita and Gibeaut, 1993) were reflected in the immunosorbence glycome arrays and linkage analyses of chelator- and alkali-soluble fractions from isolated cell walls of young Arabidopsis leaves and etiolated maize coleoptiles (Figures 2 and 3). As the Golgi apparatus is the site of synthesis and trafficking of noncellulosic components of the primary cell wall, it was expected that the polysaccharide contents of the Golgi membranes might closely reflect each type of cell wall. Whereas a full range of xylan epitopes (both backbone and side-chain epitopes) were present in the maize Golgi, linkage analyses showed that GAX hemicelluloses characteristic of Type-II walls of grasses were less abundant in the maize Golgi membranes compared with their walls. By contrast, Non-Fuc XyGs of low abundance in walls were highly represented in the Golgi (Figures 4A and 4B). The converse was true in Arabidopsis, where epitopes of both fucosylated and Non-Fuc XyGs were of low abundance in Golgi compared with the walls, and linkage analyses showed xylans to be the dominant hemicellulose of the Golgi but much less abundant in the wall (Figures 4A and 4C).
The glycome composition of the Golgi represents only a snapshot of what is made and accumulated at the time of harvest, whereas the cell wall represents the cumulative synthesis up to the point of harvest. Nevertheless, at least for maize coleoptiles, the polysaccharide composition of the Golgi are distinct with cell-wall analyses across the time-course of this organ’s growth (McCann et al., 2007). Similarly, the polysaccharide composition in Golgi of Arabidopsis seedlings are inconsistent with previous analyses of cell-wall compositions from seedlings or mature tissues (Zablackis et al., 1995).
We offer three possible explanations for these anomalous results. First, cell-wall polysaccharides that accumulate to higher abundances in Golgi might result from lower trafficking rates compared with higher rates for material that accumulates in the wall. Second, lower trafficking rates might reflect diversion of a subset of polysaccharides to lytic compartments instead of the cell wall. The TGN-Early Endosome is often distinct from the Trans-membranes of the Golgi stack and plays a central role, post-Golgi synthesis, in the sorting and packaging of polysaccharide and protein cargoes destined for the cell wall (Kang et al., 2011; Rosquete et al., 2018). Designated as the “SYP61 compartment” in its role in exocytosis (Drakakaki et al., 2012), other proteins, such as ECHIDNA (Gendre et al., 2011), and ECHIDNA/YIP4a and YIP4b complex (Gendre et al., 2013), RabA4b, and a phosphotidyl-4 kinase (Kang et al., 2011) are all implicated in trafficking through the TGN. A SCAMP2 protein is a marker for clusters of secretory vesicles that emanate from the TGN and fuse with the PM (Toyooka et al., 2009). Maize homologs of these TGN-associated proteins were not among those detected in the Golgi enriched by FFE, although we identified numerousrat sarcoma small GTPase-like proteins (Ras) and Ras binding protein (Rab)-like proteins, syntaxins, and SNARE proteins involved in vesicle trafficking and membrane fusion (Supplemental Data Set 2). Whether polysaccharide sorting occurs at the TGN to wall or lytic compartments remains to be explored.
A third possibility is that all materials are trafficked to the cell wall, but specific polysaccharides are digested extracellularly and, therefore, accumulate to much lower levels in the wall. A low abundance of AGP glycans in the cell wall is expected based on its GPI-anchored location at the exterior of the PM (Tan et al., 2012). Only miniscule amounts of Ara-containing polysaccharides in soluble fractions were centrifuged from vacuum-infiltrated pea epicotyl sections (Terry and Bonner, 1980), and treatment of these sections with auxin enhanced the yield only of XyG oligomers (Terry and Jones, 1981). Further, isolated enzymatically active cell walls yield primarily Ara and Gal by “autolysis” through action of nascent β-galactosides and α-arabinofuranosidases active against arabinogalactans (Labrador and Nicolás, 1984). Therefore, the low abundance of AGP glycans in the cell wall is more likely a result of their rapid turnover rather than their loss during homogenization of the cell-wall material.
Regardless of the mechanism by which the distribution of noncellulosic polysaccharides in the Golgi membranes is established, the larger question is why a species would place a large metabolic investment in polymers that fail to accumulate in the wall. What selective advantage might this seemingly inefficient and futile synthesis provide? One possible explanation is that maintenance of the capacity to synthesize a full range of polysaccharides serves as a hedge against mutations in synthases of cell-wall–abundant polysaccharides that could jeopardize viability. Several examples of cell-wall plasticity to maintain near-normal growth and development have been recognized. The murus1 (mur1) mutation, which results in complete loss of Fuc in the plant (Reiter et al., 1993), the mur4 and mur5 mutations, which greatly reduce the de novo synthesis of Arap and Araf, respectively (Burget et al., 2003; Dugard et al., 2016), and the rhm1(rol1) mutation, which leads to limited synthesis of rhamnose and, consequently, of RG-I (Reiter and Vanzin, 2001; Diet et al., 2006), has little effect on normal development apart from causing a more diminutive stature. From linkage analyses, mur1, mur4, and mur5 mutants demonstrate enhanced HG and/or Xyl-HG levels, and rhm1(rol1) shows enhanced arabinan and galactan branches on the residual pectin (Mertz et al., 2012; Saffer et al., 2017), modifications that might compensate for the lack of the polysaccharides containing these sugars. The low cell-wall rhamnose mur8 mutant has diminished levels of HG and RG-I but enhanced synthesis of arabinans, xylans, and XyG (Mertz et al., 2012). Cell-wall plasticity in response to mutation is also evident in the modified fine structure of a polysaccharide. For example, failure to adequately add at least one Gal residue to XyG units results in significant loss of hypocotyl tensile strength (Ryden et al., 2003; Peña et al., 2004); loss of galactosylation of the Xyl residue closest to the reducing end of the unit tetramer in mur3 results in enhanced galactosylation at the adjacent Xyl, rescuing a wild-type phenotype in this mutant (Madson et al., 2003). However, null alleles of mur3 combined with mutations in a second XyG galactosyl transferase xlt2 result in severe growth abnormalities traced to a dysfunctional XyG (Kong et al., 2015; Xu et al., 2017). The Arabidopsis xxt1 xxt2 double mutant completely lacks XyG in the wall, but despite a slight lowering of tensile strength, plant structure is remarkably unchanged from wild type (Cavalier et al., 2008; Zabotina et al., 2012). Enhanced synthesis of xylan, and possibly HG, provide a possible mechanism of recovery of viable plants. Perhaps the starkest example of plasticity is the survival of cells in liquid culture in the near absence of cellulose induced by a potent cellulose synthesis inhibitor (Shedletzky et al., 1990). Tomato (Solanum lycopersicum) and tobacco (Nicotiana tabacum) cells with Type-I walls respond by increased cross-linking of a pectin matrix, whereas barley (Hordeum vulgare) cells with Type-II walls enhance xylan deposition and cross-linking of their phenylpropanoid networks (Shedletzky et al., 1992).
With the lone exception of AG-2 mAbs CCRC-M133 and CCRC-M107, which have strong signals for epitopes in the maize cell wall, epitopes of other AG-2, RG-I/AG, and AG-4 types were of low abundance in the maize cell wall and more abundant in Golgi compared with the cell wall in both species (Figure 4). In a comprehensive survey of synthetic epitopes of this class, Ruprecht et al. (2017) found that the majority of the mAbs in the RG-I/AG class bind to 3- and 6-linked galactans. Coupled with the fact that none of the mAbs that bind to RG-I backbones showed binding in the Golgi, these galactan epitopes were more likely to be on AGPs. This inference was confirmed by linkage analysis, showing that maize Golgi had very little RG-I and HG compared with AGPs and 5-arabinans (Figure 4B). This contrast indicates that these epitopes are associated with Golgi-resident proteoglycans or that their synthesis, flux, and turnover rates are substantially higher than for exported pectins and hemicelluloses that accumulate in the cell wall. The presence of AGPs as the major proteoglycans of the Golgi membranes of both species might indicate a common role in facilitating synthesis and trafficking of wall matrix polymers. In Arabidopsis, the AGP family member Arabinoxylan Pectin Arabinogalactan Protein1 (APAP1) contains glycan structures linked to xylan and RG-I residues and could therefore represent a structural component in the cell wall (Tan et al., 2013). However, an alternative possibility is that core structures similar to APAP1, or other fasciclin-like AGPs, synthesized in the Golgi could serve as initiators of pectin and hemicellulose synthesis or glycochaperones during trafficking to the cell wall and turnover upon delivery of their cargo. Our findings are focused on primary wall formation during active growth of young plant organs, and a remaining question is whether Golgi polysaccharide composition is equally as broad in cells of mature tissues, and during secondary wall formation when fewer types of cell-wall constituents accumulate.
The Maize Golgi Proteome
The separation by FFE of membranes previously enriched by flotation centrifugation enabled detection of >2,000 proteins associated with at least one of 12 collected fractions. Of these, 230 proteins were known to be associated with synthesis or metabolism of the cell wall, more than doubling the number reported previously using conventional downward centrifugation of Arabidopsis or Italian ryegrass (Lolium multiflorum) membranes (Nikolovski et al., 2014; Ford et al., 2016).
Owing to a genome duplication event approximately 11 million years ago (Gaut and Doebley, 1997), maize gene families are larger, with more gene isoforms expressed at any particular stage of development (Penning et al., 2009). For example, maize CesA genes number twenty (20), double the number in Arabidopsis and rice. Sixteen CesAs are expressed in the maize coleoptile; of these, 12 isoforms were detected by either MaxQuant (www.maxquant.org) or Mascot (Matrix Science; Supplemental Data Set 4). The expansion of the number of maize CesAs extends well beyond the classical three isoforms described for Arabidopsis primary and secondary wall synthesis (Taylor, 2008), suggesting that multiple combinations of isoforms might contribute to synthase complexes.
Consistent with the detection of extraordinarily high amounts of pectin/AGPs in Golgi, proteins associated with their synthesis were numerous. Eleven of 12 expressed isoforms of family GT8D UDP-GalA transferases associated with HG synthesis were identified, as well as six fasciclin-like AGPs, eight prolyl 4-hydroxylases that synthesize Hyp residues for O-glycosylation, and eight family GT31 UDP-Gal transferases associated with AGP galactan synthesis (Supplemental Data Set 4). In addition to the fasciclin-like proteins associated with AGPs, several GPI-anchored proteins were identified, including two COBRA proteins, a glycerophosphodiesterase, a Lysine motif (LysM)-chitin elicitor receptor kinase4c, a SKU3, and three phospholipid transfer proteins (Supplemental Data Set 4). Borner et al. (2002) provided bioinformatic prediction of GPI-anchors for these and other proteins, as well as proteomic support for the association of these proteins with microsomal membranes (Borner et al., 2003).
Three family GT14 β-d-glucuronosyl transferases known to decorate the AG chains were also detected. As GAX is the major cell-wall polysaccharide of grasses, a rich assembly of proteins involved in backbone synthases and glycosyl transferases for GAX were detected, including eight IRX10-like family GT47E xylosyl transferases (IRX10-L1 isoforms) involved in chain synthesis, seven members of family GT43 (IRX9 and IRX14 proteins) involved in backbone elongation, and eight GT61 glycosyl transferases implicated in xylosylation and arabinosylation of the xylan backbone.
The appearance of Non-Fuc XyGs in high abundance in the maize Golgi was surprising, considering its low abundance in the cell wall (Figures 4A and 4B). The capacity for XyG synthesis was validated by detection of several isoforms of XyG (1→4)-β-d-glucan backbone synthases (CslCs) and XyG-specific (1→6)-α-d-xylosyl transferases (XXTs). Grass XyGs are generally less frequently branched with Xyl and Gal residues (Scheller and Ulvskov, 2010), but despite the reduced XyG galactosylation in grasses, three GT47A XyG α-galactosyl transferases, including two MUR3 homologs (Madson et al., 2003), were detected (Supplemental Data Set 4). A Fucosyl Transferase1 (FUT1) homolog of MUR2 (Vanzin et al., 2002) was also detected, indicating that maize Golgi have the capacity to make fucosylated XyGs common among most angiosperms. Despite lack of detectable epitopes of fucosylated XyGs in Golgi, several epitopes associated with fucosylated XyGs were weakly detected, and one strongly detected, in the maize cell wall. Although uncommon in grasses, fucosylated XyGs have been detected in certain tissues (Brennan and Harris, 2011; Liu et al., 2015). In a complementary study of Arabidopsis, cell-wall–related proteins in Golgi isolated by FFE, synthases, and glycosyl transferases were detected for the full range of cell-wall polysaccharides (Parsons et al., 2012). The expected CslC XyG β-Glucan Synthases, XXTs, MUR2, and MUR3 enzymes for fucosylated XyGs, the numerous GT8D UDP-GalA Transferase enzymes for HG synthesis, putative rhamnosyl transferases for RG-I synthesis, and the Arabinan Deficient1 (ARAD1) arabinosyl transferase for 5-arabinan synthesis were detected, as were IRX10, IRX14, GUX3, and XYLT for xylan backbone synthesis and xylosyl side-groups (Supplemental Table 7).
Asymmetric Protein Distribution in Maize Golgi Membranes after FFE
Whereas the majority of the Golgi-associated enzymes of nucleotide-sugar interconversion are more or less uniformly distributed across the four Golgi fractions (Figure 7C), the vast majority of proteins detected after FFE were asymmetrically distributed into three major fractions of bulk Golgi, a fraction rich in CesAs, and a leading shoulder of ER. Golgi bodies comprise several development stages, from fusion of COPII vesicles to form the cis-membranes to their transition through medial- and Trans-membranes to the TGN (Donohoe et al., 2013; Uemura et al., 2014). Location of synthase complexes has been inferred by immunocytochemistry for their products, where XyGs localize to Trans-Golgi cisternae and the TGN, pectic polysaccharides to the cis-, medial-, and Trans-Golgi cisternae (Zhang and Staehelin, 1992), and xylogalacturonan to early Trans-membranes versus XyG and HG/RG-I to late Trans-membranes and the TGN (Wang et al., 2017). The observation that synthases and glycosyl transferases of pectin, AGP, GAX, and XyG synthesis follow the bulk Golgi pattern indicates that these Golgi maturation stages are not differentiated.
The Golgi membrane has been shown to be the site of assembly of cellulose synthase complexes by freeze-fracture electron microscopy (Haigler and Brown, 1986) and live-cell imaging of fluorescence-labeled CesA proteins (Crowell et al., 2009; Gutierrez et al., 2009). All but one of the CesA proteins identified in Golgi formed a unique pattern that peaked at F31, coincident with a small spike of abundance in the Golgi peak identified during FFE (Figures 7A and 7E). The colocalization of the CesA proteins in this fraction indicated a possible concentration of the Small CesA compartment (SmaCC) or Microtubule-associated CesA compartment (MASC) small vesicles that are transported to the PM (Crowell et al., 2009; Gutierrez et al., 2009). However, KOR and COBRA proteins, known to be associated with CesAs (Liu et al., 2013; Vain et al., 2014), followed the bulk Golgi pattern, and a DUF288-containing STELLO protein that interacts with CesAs in the Golgi (Zhang et al., 2016) was abundant in all four fractions (Figure 7F).
The CslF proteins form at least part of the synthase complex for the mixed-linkage β-glucan (Doblin et al., 2009). Although substantial data demonstrate the Golgi-associated synthesis of the mixed-linkage β-glucan in vitro and in vivo (Gibeaut and Carpita, 1993; Buckeridge et al., 1999; Urbanowicz et al., 2004; Carpita and McCann, 2010; Kim et al., 2015), others have suggested that at least some CslF synthases of this polysaccharide traffic to the PM, where synthesis continues or even commences in muro (Wilson et al., 2015). Indeed, a CslF5 showed enrichment in the CesA pattern (Figures 7E and 7F), and not with the general pattern of pectin and hemicellulose synthases (Figure 7D). Thus, an association of CslF proteins with CesA transport to the PM is possible.
CONCLUSION
A combination of glycome and linkage analyses revealed that the Golgi apparatus of two angiosperm species with distinctively different cell walls possess a common carbohydrate matrix of AGPs and pectins. Further, epitopes and linkages of the full range of polysaccharides made by both species were enriched to unexpected degrees. Isolation of maize Golgi membranes by flotation centrifugation, followed by separation by FFE, provided a rich inventory of resident and transiting proteins of the Golgi apparatus, including synthases and glycosyl transferases for a broader range of polysaccharides than expected based on wall composition. The capacity of grass species to make a full repertory of polysaccharides opens up new strategies for altering and engineering cell-wall composition of grasses and other bioenergy crop species. The identification of a CesA-rich fraction distinct from the bulk Golgi membranes involved in synthesis of noncellulosic polysaccharides offers opportunities for further purification of the synthase complex.
METHODS
Plant Materials
Seeds from maize (Zea mays) were soaked in the dark for ∼12 h at ambient temperature in deionized water bubbled with air, then sown in moistened medium-grade vermiculite and grown in darkness for 2.5 to 3 d. Seeds of Arabidopsis (Arabidopsis thaliana) Columbia-0 (Col-0) were soaked in deionized water for 3 d at 4°C to prime germination and sown in 4″ pots containing Metromix360 (Sungro). They were then grown in growth chambers in a 16-h–light/8-h–dark photoperiod at 23°C. Light at 150 μmole m–2 s–1 Photosynthetic Active Radiation (PAR) at benchtop was delivered by VYPR 2× lamps (Fluence Bioengineering), with spectral distribution available at https://fluence.science/store/vypr-series/vypr-2×/. The seedlings were grown for 20 to 22 d before harvest.
Isolation of Crude Cell Walls and Golgi Membrane Fractions
In triplicate independent experiments, 40 g of fresh Arabidopsis leaves at 21 ± 1 d and 60 g of maize coleoptiles (and etiolated leaves within) at 2.5 to 3 d were harvested into a chilled mortar, and overlaid with an equal volume of ice-cold homogenization buffer consisting of 100-mM HEPES (1,3-bis[tris(hydroxymethyl)-methylamino]propane at pH 7.4), 20 mm of KCl, 40 mM of ascorbic acid, and 84% (w/v) Suc (Figures 1A and 1B; Supplemental Figure 1). The isolated coleoptiles were stirred gently in homogenization buffer for ∼5 min before mashing in a chilled mortar and pestle (Gibeaut and Carpita, 1990, 1994). The homogenates were squeezed through a nylon mesh (45-μm2 pores) and adjusted to ∼42% (w/v) Suc. Cell walls retained by the nylon mesh were directed to isolation as described below. To isolate Golgi membranes, 20 mL of the homogenate was pipetted into each 38.5-mL centrifuge tube (Ultraclear; Beckman) and overlaid with 7 mL each of 35%, and 29% (w/v) Suc, and 4 mL of 18% (w/v) Suc in a gradient buffer containing 20-mM HEPES [Bis Tris-propane], pH 7.6, and the remaining volume was made up with 9.5% (w/v) Suc in the same buffer. After flotation centrifugation at 27,000 rpm (131,500 × g at rmax) in an SW28 rotor (Beckman) for 90 min at 4°C, the interface containing Golgi membranes (29%/35%) was collected with a wide-bore plastic Pasteur pipette, and used directly in FFE. Other fractions of the Golgi membranes and their proteins were precipitated using 5 vol (v/v) of cold (–20°C) 100% acetone, and held at –20°C overnight. After five washes with excess chilled 80% acetone (–20°C), the Suc-free pellet was air-dried and suspended in 100 μL of distilled water for glycome array and linkage analyses, and for proteome analysis as “pre-FFE” proteins. A flow chart for materials is provided in Supplemental Figure 1.
Preparation of Cell-Wall Materials
Cell-wall materials from the three independent harvests of Arabidopsis leaves and maize coleoptiles after membrane isolation were washed several times in water and then ground in 1% (w/v) SDS in 50 mM of Tris[HCl], pH 7.2. Samples were then incubated to 60°C and washed sequentially in 1% (w/v) SDS-containing grinding buffer, methanol at 50°C for 20 min, and then 50% (v/v) ethanol. Supernatants were discarded to remove soluble compounds and the pellet was finally washed with Barnstead GenPure water (Thermo Fisher Scientific). Starch was extracted from the pellet by sonication in 90% (v/v) DMSO in water (Carpita and Kanabus, 1987). Cell walls were then washed twice with distilled water and suspended in distilled water at 4°C overnight.
Fractionation of Cell-Wall Polysaccharides
Three independent sequential extractions of isolated cell-walls’ materials were performed with 0.5% (w/v) ammonium oxalate (pH 7.0) at 90°C, then 0.1-M, 1-M, and 4-M NaOH (each containing 3 mg mL–1 of sodium borohydride) at ambient temperature with continuous stirring. Each extraction was done twice, with 20 mL and 10 mL of the appropriate reagent for 1 h and 30 min, respectively, and then common extracts were pooled. The second extraction with 4-M NaOH was done overnight before pooling. After each extraction period, the samples were centrifuged at 4,500 rpm (3,900 × gmax), and the next reagent added to the pelleted wall material. Pooled supernatants were filtered through glass fiber filter discs, chilled and neutralized with acetic acid, dialyzed extensively against water, and freeze-dried. Total sugar in each fraction was estimated by phenol-sulfuric assay (DuBois et al., 1956) using standards for the most abundant neutral sugars, Glc, Ara, Xyl, and Gal, and for GalA. Alditol acetate derivatives of carboxyl-reduced materials were used to determine the monosaccharide mole % distributions, and therefore total milligrams of carbohydrate for loading in glycome profiling. Total monosaccharide for each fraction of noncellulosic material was used to estimate the relative proportions in the wall based on relative recovery in each fraction.
Glycome Profiles of Golgi and Cell-Wall Polysaccharides
Three independent preparations of acetone-washed Golgi pellets and freeze-dried cell-wall fractions were suspended in Barnstead GenPure water (Thermo Fisher Scientific) water and sonicated for 30 min in a water bath, and then centrifuged at 4,500 rpm (3,900 × gmax) to sediment a small amount of undissolved material. The supernatant was brought to ∼400 µg mL–1 total carbohydrate. The small amounts of Golgi material prevented estimation of sugar amounts, and intensities were normalized to the total intensities of the combined cell-wall fractions. The glycomics assay of the cell wall and Golgi fractions followed the ELISA-based procedure as described by Pattathil et al. (2012). Briefly, the cell-wall extracts are probed against a comprehensive suite of 155 plant cell-wall glycan-directed mAbs described in Pattathil et al. (2010) and as updated in Pattathil et al. (2015), monitoring most major noncellulosic plant glycan epitopes using ELISAs. ELISA plates (Costar 3700; Corning) were coated on an equal carbohydrate basis (0.3 μg/well), and assays were done in two technical replicates using a Robotic system (Thermo Fisher Scientific). Water was used as the blank to assess background values, which were subtracted from each sample reading. The mean background-subtracted absorbance values (OD450–655nm) were then represented as heat maps using a modified version of R-console software (Pattathil et al., 2012). The collection of mAbs employed were obtained from laboratory stocks (CCRC, JIM, and MAC series) at the Complex Carbohydrate Research Center (available through CarboSource Services; http://www.carbosource.net) or from BioSupplies (BG1, LAMP). Detailed information about the mAbs used can be found in the WallMabDB database online (www.wallmabdb.net). Absorbance values for each epitope of the noncellulosic polysaccharides extracted by each of the ammonium oxalate and alkali extracts were scaled by their weight % contribution to mass to obtain an estimate of the relative distribution of total pectic and hemicellulosic epitopes in the total cell wall.
Monosaccharide and Linkage Analysis
Uronic acids in a 3-mL suspension of the Golgi and the ammonium oxalate- and 0.1-M NaOH cell-wall fractions were reduced by activation with 0.25 g of 1-cyclohexyl-3-(2-morpholinyl-4-ethyl) carbodiimide (methyl-p-toluene sulfonate) followed by addition of 300 mg of sodium borodeuteride, and then supplemented with 0.5 mL of 2 M imidazole (HCl) at pH 7, to prevent alkali degradation, as described in Kim and Carpita (1992), and as modified by Carpita and McCann (1997). After 2 h of incubation, the solution was acidified with glacial acetic acid to destroy excess borohydride and then dialyzed extensively against Barnstead GenPure water (Thermo Fisher Scientific) water and lyophilized.
Monosaccharide composition was determined essentially as described by Gibeaut and Carpita (1991). Approximately 1 to 2 mg of lyophilized Golgi and cell-wall samples were hydrolyzed with 1 mL of 2-M trifluoroacetic acid (TFA; containing 1 μmole of myo-inositol as internal standard) for 90 min at 120°C with vortex mixing every 30 min. The TFA was evaporated under a stream of dry air at 40°C to 45°C. Sugars were then reduced with 20 mg mL–1 of sodium borohydride (NaBH4) in DMSO containing 0.2 M of NH4OH and incubated at 45°C for 90 min with vortexing every 30 min. The solution was neutralized with 100 μL of glacial acetic acid, and 100 μL of 1-methyl-imidazole added, followed by addition of 0.75 mL of acetic anhydride. The mixture was incubated at 45°C for 30 min, and then 1.5 mL of water added to destroy excess acetic anhydride. After cooling, the alditol acetates were partitioned into dichloromethane and then separated by GLC on a 0.25-mm × 30-m column of SP-2330 (Supelco). Injection was at 80°C with a 1-min hold, ramped to 170°C at 25°C min–1, and then to 240°C at 5°C min–1, with a 10-min hold at the upper temperature. Helium flow was 1 mL min–1 with split-less injection. The electron impact mass spectrometry was performed with a model no. 5973N MSD (Agilent) at 70 eV and a source temperature of 250°C.
Linkage analysis was done as described in Gibeaut and Carpita (1991). Briefly, silylation-grade DMSO was added by syringe to ∼1 mg of lyophilized Golgi and cell-wall samples in a tube sealed with a serum stopper, and the tubes were sonicated for 90 min until the water bath warmed to 50°C. n-Butyllithium (2.5 M) in hexane was added dropwise to the DMSO suspension with rapid stirring while the sample was purged with Argon, and the solution was stirred gently for ∼1 h for evaporation of hexane and complete formation of the alkoxide ions. Iodomethane was then added drop-wise to the stirred solution until neutralized. The reaction was quenched with water, the partly methylated polymers were partitioned into chloroform, and the chloroform phase was washed five times with water and dried under nitrogen gas. The partly methylated polymers were then hydrolyzed in 2 M of TFA for 90 min at 120°C, reduced with sodium borodeuteride (NaBD4), and acetylated. GLC-electron impact MS was performed as described in Gibeaut and Carpita (1991), and the linkage structure was inferred as described in Carpita and Shea (1989).
Visualization of Maize Golgi Membranes
Structural integrity and enrichment of the Golgi membranes from flotation centrifugation was confirmed by TEM. Briefly, 0.25 mL of Golgi membrane fraction was fixed by addition of 25 μL of 25% (v/v) glutaraldehyde to give a final concentration of 2.5%. After 30 min, samples were then diluted slowly with additional 2.5% (v/v) glutaraldehyde in 0.1 M of KH2PO4 at pH 7.2, to 1.5 mL. Pellets were collected after centrifugation for 30 min and resuspended in fresh 2.5% (v/v) glutaraldehyde for another 1 h. Fixed samples were rinsed in buffer, postfixed in buffered 1% (w/v) osmium tetroxide containing 0.8% (w/v) potassium ferricyanide, rinsed again in distilled water, and embedded in agarose. The membranes in agarose were stained en bloc with 1% (w/v) uranyl acetate, dehydrated with a graded series of ethanol to 100%, and transferred into acetonitrile and embedded in EMbed-812 Resin (Thermo Fisher Scientific). Thin sections were cut on a model no. Ultracut E Ultramicrotome (Reichert-Jung) and stained with 4% (w/v) uranyl acetate and lead citrate. Images were acquired on a Tecnai G2 T12 Electron Microscope equipped with a tungsten source (FEI) and operating at 80 kV.
FFE
FFE of the three independent maize Golgi membrane preparations collected after flotation centrifugation was performed essentially as described in Parsons et al. (2012, 2014). Briefly, a Separation Buffer consisted of 10 mM of sodium acetate at pH 7.0, supplemented with 280 mM of Suc, 10 mM of triethanolamine, 1 mM of EDTA, and a Stabilization Buffer was made by adjusting the Separation Buffer to 100 mM of sodium acetate at pH 6.5, with 200 mM of Suc, 100 mM of triethanolamine, and 10 mM of EDTA. Anodic and cathodic electrode buffers were 100 mM of sodium acetate at pH 6.5, with 100 mM of triethanolamine and 10 mM of EDTA. Voltages of 680 to 700 V were applied to the membrane samples, resulting in a current of 132 to 137 mA. Media flow rate was set to 250 mL h–1, and sample flow rate to 2.5 mL h–1. Fractions were collected in cooled 2-mL 96-well plates, and fractions of interest identified after detection at 280 nm were pooled into four fractions. Membranes were collected from fractions by centrifugation at 50,000 × g for 45 min, resuspended in ice-cold 10-mM Tris-HCl at pH 7.5, and stored at –80°C.
Analysis of the Golgi Membranes by MS/MS
Proteins isolated from the fractions enriched for Golgi membranes after flotation and FFE separation were reduced for disulfide bonds in 10 mM DTT, Cys-alkylated in 20 mM of iodoethanol, and then digested with trypsin (1:10 w/w) overnight at 37°C in 40% (v/v) methanol and 10 mM of Tris(HCl) at pH 7.8. After overnight digestion, samples were dried in a SpeedVac concentrator. Digested peptides were desalted using a Pierce C18 spin column (Thermo Fisher Scientific) before LC-MS/MS analysis using the manufacturer’s protocol. Peptide samples were rehydrated in a solution of 2% (v/v) acetonitrile and 0.1% (v/v) formic acid. The samples were run on a Nano Eksigent 425 HPLC system (Sciex) coupled to the Triple TOF 5600 Plus (Sciex). LC-MS/MS data were collected using 120 min of LC gradient at 300 nL/min over the cHiPLC Nanoflex System (Sciex). The trap column was a Nano cHiPLC 200-μm × 0.5-mm ChromXP C18-CL (Sciex), 3 μm, 120 Å followed by the analytical column, the Nano cHiPLC 75-μm × 15-cm ChromXP C18-CL (Sciex), 5 μm ,120 Å. The sample was injected into the Triple TOF 5600 Plus through the Nanospray III ion source equipped with an emission tip (New Objective). Peptides from the digestion were eluted from the columns using a mobile phase A of purified water/0.1% formic acid and a mobile phase B of acetonitrile/0.1% formic acid. With a flow rate of 0.3 μL min–1, the method started at 95% A for 1 min was followed by a gradient of 5% B to 35% B in 90 min and from 35% B to 80% B in 2 min. The run was held at 80% B for 5 min before being brought to 5% B and held for 20 min. The data acquisition was performed monitoring 50 precursor ions at 250 ms per scan.
LC-MS/MS Data Analysis
The raw data (.wiff) were processed using the software MaxQuant( version 1.6.0.16) with its integrated Andromeda search engine (Cox and Mann, 2008; Cox et al., 2014) and the software Mascot (version 2.3.02; Matrix Science) and searched against our custom Maize annotation database (125,561 sequences; 39,895,172 residues) comprising proteins from Maize version 2 WGS (www.maizeGDB.org) using the largest representative transcript of each gene in the case of multiple models (https://www.maizegdb.org/gbrowse/maize_v2test?q=Chr1:1..301354135;label=CellWallGenes) and the common Repository of Adventitious Proteins (cRAP version 1.0, The Global Proteome Machine). This maize annotation resource is also summarized and posted at our Cell Wall Genomics website (https://cellwall.genomics.purdue.edu/families/index.html). Mascot was set to search with the following parameters: peptide tolerance = ±0.05 Da; MS/MS tolerance = ±0.2 Da; fixed modification with Ethanolyl (C); variable modifications with Acetylation (K) and Oxidation (M), for one missed cleavage for trypsin; instrument type set to “ESI-QUAD-TOF.” For both searches, the “decoy” search option was activated to determine the False Discovery Rate (FDR) of peptides and proteins. Mascot peptide matches were accepted if the significance scores of their match had a P value ≤ 0.05 and a peptide score ≥ 13. Mascot search results were filtered further to accept peptides with rank 1 and a significance homology threshold of ≤1% FDR. MaxQuant search was also set at 1% FDR at both peptide spectrum match and protein levels. The minimum peptide length required for database search was set to six amino acids. Other parameters applied for MaxQuant search include: precursor mass tolerance = ±0.05 Da; MS/MS fragment ions tolerance = ±0.2 Da; maximum missed cleavage for tryptic digestion = 1; Met oxidation and acetylation (K) were set as the variable; Ethanolyl (C) was set as a fixed modification. Application of Pro hydroxylation as a variable modification in either Mascot or MaxQuant identified no additional Hyp-containing glycoproteins.
Postsearch Data Filtering
Proteins identified as false hits and contaminants, identified from MaxQuant decoy and contaminants databases, were filtered out. For each experiment, proteins with negative scores and those with a total MS/MS count of zero across the four fractions were filtered. Thus, for a protein to be counted as identified in each experiment, it must have been present in at least two fractions. However, proteins in one fraction with MS/MS counts >1, with supporting intensity data and peptide counts, were considered valid hits. When proteins across experiments were combined, those with an average MS/MS count of ≥1 in at least two experiments were considered as identified.
If the same peptides are mapped to multiple proteins, the softwares Mascot and MaxQuant combines these proteins into a protein group or protein family. For effective accounting of cell-wall–related proteins, we called out every protein from each cell-wall–related protein group in MaxQuant results that had a corresponding RNA expression value. The MS/MS counts assigned to a specific group of cell-wall proteins was shared among the proteins called out from the group using the RNA expression value as a measure of distribution. When RNA expression was low for a particular protein called out from a group, no MS/MS value was assigned. Protein groups with low MS/MS counts were assigned to the protein with the highest transcript reads, and the remainder were unassigned.
For the Mascot data, proteins with peptide expectation values ≤ 0.05 were selected in each experiment. Proteins were counted as identified if total spectral counts were ≥1 and found in at least two out of the four fractions, or ≥5 in a single fraction. For isoforms of some cell-wall–related proteins that could not be resolved by Mascot, but were detected by MaxQuant and have expression support, we denoted them as “unassigned.”
Asymmetric Distribution of Proteins
For the enrichment analysis, the total pre-FFE and post-FFE abundance from the filtered Mascot data were used because they represented a more comprehensive proteome data set. For both the pre-FFE and post-FFE Golgi results, spectral counts for each nonredundant protein were summed to get total counts for both, and this value used for normalization against the highest total spectral count between them. For determination of asymmetric distribution of different classes of cell-wall proteins, we pooled the protein data from the three biological replicates of maize Golgi preparations across the four FFE fractions. Abundance of each protein across the four Golgi fractions was relative to the fraction with highest abundance (1.0). Proteins with high abundance and identified with high scoring peptides (expectation values ≤ 0.05 and scores ≥ 10) were generally used to determine the distribution.
Maize Coleoptile Gene Expression
Three batches of etiolated maize coleoptiles (and etiolated leaves within), harvested at 2-, 2.5-, and 3-d postplanting, were excised aseptically from the internodes and immediately plunged in liquid N2. Samples in triplicate were kept frozen and pulverized by mortar and pestle under liquid N2. Approximately 2 mg of ground tissue was incubated with 1 mL of ice-cold TRIzol reagent (Thermo Fisher Scientific) and extracted according to the manufacturer’s directions. Purified RNA was dissolved in 50 μL of diethyl pyrocarbonate-treated Barnstead GenPure water (Thermo Fisher Scientific) water and quality and concentration were determined spectrophotometrically.
Expression analysis was performed as described in Penning et al. (2014). Briefly, the pooled RNA samples from the three biological replicates were delivered to Purdue’s Genomics Core facility for RNA-Seq using an Illumina HiSeq 2000 to process 100-bp × 100-bp libraries of ∼400-bp inserts. High quality trimmed sequences were mapped to the Maize B73 sequence V2 from Plant GDB (http://www.plantgdb.org) using the software Bowtie2 (Langmead and Salzberg, 2012) except in instances where the reads mapped to exactly two places in the genome due to gene duplication in maize. For these, a custom Perl script was used to split the reads between the two locations (Penning et al., 2014). An average mapping rate of 80% was achieved over all samples. The counts for each tissue were appended to an Excel (Microsoft) readable file with maize gene names and locations. Total reads from the RNA-Seq analyses of the three sampling times were pooled and normalized to 20-M reads.
Accession Numbers
Our updated maize B73 annotations of cell-wall–related genes are available at Cell Wall Genomics (https://www.maizegdb.org/gbrowse/maize_v2test?q=Chr1:1..301354135;label=CellWallGenes). Detailed information about the mAbs used can be found in the WallMabDB database (www.wallmabdb.net). The MS proteomics data have been deposited to the ProteomeXchange Consortium via the partner repository (Perez-Riverol et al., 2019) with the data set identifier PXD007612 and null. The RNA-Seq data are available at NCBI (https://www.ncbi.nlm.nih.gov/sra/PRJNA522447).
Supplemental Data
Supplemental Figure 1. Flow chart for the isolation and enrichment of arabidopsis and maize Golgi membranes and cell-wall pectic and hemicellulosic material.
Supplemental Figure 2. Panel of 155 mAbs employed in glycome analysis.
Supplemental Figure 3. Reproducibility and correlation of MaxQuant and mascot analyses of maize Golgi proteins enriched by FFE.
Supplemental Figure 4. Distribution of maize ER-Golgi proteins identified and quantified by MaxQuant MS/MS counts after enrichment of Golgi by FFE.
Supplemental Table 1. Diagnostic linkage groups determined by GLC-electron-impact MS of partly methylated alditol acetate derivatives of polysaccharides.
Supplemental Table 2. Linkage composition of arabidopsis cell-wall fractions after sequential extractions with ammonium oxalate, and increasing concentrations of alkali, and of Golgi membranes after flotation centrifugation.
Supplemental Table 3. Linkage composition of maize cell-wall fractions after sequential extractions with ammonium oxalate, and increasing concentrations of alkali, and of Golgi membranes after flotation centrifugation.
Supplemental Table 4. Arabidopsis Golgi-associated proteins identified after FFE (from Parsons et al., 2012).
Supplemental Data Set 1. Cell-wall–associated proteins in Golgi preparations from etiolated maize coleoptiles after flotation centrifugation.
Supplemental Data Set 2. Total non-cell–wall Golgi proteins quantified as MaxQuant MS/MS counts and mascot spectral counts after FFE.
Supplemental Data Set 3. Comparison of annotated maize GDB descriptions compared with those of phytozome for maize cell-wall–related families of proteins identified in Golgi membranes isolated by FFE.
Supplemental Data Set 4. Total cell-wall proteins in Golgi membrane preparations from etiolated maize coleoptiles after FFE, quantified as MaxQuant MS/MS counts and mascot spectral counts compared with transcript abundance.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Vicki Hedrick (Proteomics Facility, Purdue) for her technical expertise in proteome analysis, and Christopher Gilpen (LifeScience Microscopy Facility, Purdue) for assisting in the TEM studies of membrane fractions. We thank Jyothi Thimmapuram and Ketaki Bhide (Bioinformatics Core, Purdue) for their help in analysis and submission of the RNA-Seq data. This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences (DE-SC0000997), the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research for the Golgi isolation, linkage analyses, proteomics analyses and RNA-seq experiments. The Lawrence Berkeley National Laboratory and the U.S. Department of Energy (DE-AC02-05CH11231) supported the FFE experiments. The National Science Foundation Plant Genome Program (DBI-0421683 and IOS-0923992) supported generation of the CCRC series of plant cell-wall glycan-directed mAbs used in this work, and the U.S. Department of Energy-Funded Center for Plant and Microbial Complex Carbohydrates (DE-SC0015662) for the immunological screening of cell-wall and Golgi samples.
AUTHOR CONTRIBUTIONS
I.O.O., S.P., J.L.H., M.G.H., and N.C.C. designed research; I.O.O., S.P., S.M.G.F-N., U.K.A., B.W.P., J.L., and N.C.C. performed experiments; I.O.O., S.P., U.K.A., B.W.P., J.L.H., M.G.H., M.C.M., and N.C.C. analyzed data; I.O.O., S.P., U.K.A., B.W.P., J.L.H., M.G.H., M.C.M., and N.C.C. wrote the article.
References
- Balch W.E., Dunphy W.G., Braell W.A., Rothman J.E. (1984). Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of n-acetylglucosamine. Cell 39: 405–416. [DOI] [PubMed] [Google Scholar]
- Borner G.H.H., Sherrier D.J., Stevens T.J., Arkin I.T., Dupree P. (2002). Prediction of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A genomic analysis. Plant Physiol. 129: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner G.H.H., Lilley K.S., Stevens T.J., Dupree P. (2003). Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiol. 132: 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudart G., Jamet E., Rossignol M., Lafitte C., Borderies G., Jauneau A., Esquerré-Tugayé M.-T., Pont-Lezica R. (2005). Cell wall proteins in apoplastic fluids of Arabidopsis thaliana rosettes: Identification by mass spectrometry and bioinformatics. Proteomics 5: 212–221. [DOI] [PubMed] [Google Scholar]
- Brennan M., Harris P.J. (2011). Distribution of fucosylated xyloglucans among the walls of different cell types in monocotyledons determined by immunofluorescence microscopy. Mol. Plant 4: 144–156. [DOI] [PubMed] [Google Scholar]
- Buckeridge M.S., Vergara C.E., Carpita N.C. (1999). The mechanism of synthesis of a mixed-linkage (1→3),(1→4)-β-d-glucan in maize. Evidence for multiple sites of glucosyl transfer in the synthase complex. Plant Physiol. 120: 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burget E.G., Verma R., Mølhøj M., Reiter W.-D. (2003). The biosynthesis of l-arabinose in plants: Molecular cloning and characterization of a Golgi-localized UDP-d-xylose 4-epimerase encoded by the MUR4 gene of Arabidopsis. Plant Cell 15: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffall K.H., Mohnen D. (2009). The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344: 1879–1900. [DOI] [PubMed] [Google Scholar]
- Carpita N.C., Gibeaut D.M. (1993). Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3: 1–30. [DOI] [PubMed] [Google Scholar]
- Carpita N.C., Kanabus J. (1987). Extraction of starch by dimethyl sulfoxide and quantitation by enzymatic assay. Anal. Biochem. 161: 132–139. [DOI] [PubMed] [Google Scholar]
- Carpita N.C., McCann M.C. (1997). Some new methods to study plant polyuronic acids and their esters. In Townsend R., Hotchkiss A., eds, Glycobiology. Marcell Dekker, New York, pp. 595–611. [Google Scholar]
- Carpita N.C., McCann M.C. (2010). The maize mixed-linkage (1→3),(1→4)-β-d-glucan polysaccharide is synthesized at the Golgi membrane. Plant Physiol. 153: 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N.C., Shea E.M. (1989). Linkage structure by gas chromatography-mass spectrometry of partially-methylated alditol acetates. In Biermann C.J., McGinnis G.D., eds, Analysis of Carbohydrates by GLC and MS. CRC Press, Boca Raton, FL, pp. 155–216. [Google Scholar]
- Carpita N.C., Defernez M., Findlay K., Wells B., Shoue D.A., Catchpole G., Wilson R.H., McCann M.C. (2001). Cell wall architecture of the elongating maize coleoptile. Plant Physiol. 127: 551–565. [PMC free article] [PubMed] [Google Scholar]
- Cavalier D.M., Lerouxel O., Neumetzler L., Yamauchi K., Reinecke A., Freshour G., Zabotina O.A., Hahn M.G., Burgert I., Pauly M., Raikhel N.V., Keegstra K. (2008). Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Mann M. (2008). MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26: 1367–1372. [DOI] [PubMed] [Google Scholar]
- Cox J., Hein M.Y., Luber C.A., Paron I., Nagaraj N., Mann M. (2014). Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13: 2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell E.F., Bischoff V., Desprez T., Rolland A., Stierhof Y.D., Schumacher K., Gonneau M., Höfte H., Vernhettes S. (2009). Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21: 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallabernardina P., Ruprecht C., Smith P.J., Hahn M.G., Urbanowicz B.R., Pfrengle F. (2017). Automated glycan assembly of galactosylated xyloglucan oligosaccharides and their recognition by plant cell wall glycan-directed antibodies. Org. Biomol. Chem. 15: 9996–10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Michele R., McFarlane H.E., Parsons H.T., Meents M.J., Lao J., González Fernández-Niño S.M., Petzold C.J., Frommer W.B., Samuels A.L., Heazlewood J.L. (2016). Free-flow electrophoresis of plasma membrane vesicles enriched by two-phase partitioning enhances the quality of the proteome from Arabidopsis seedlings. J. Proteome Res. 15: 900–913. [DOI] [PubMed] [Google Scholar]
- Diet A., Link B., Seifert G.J., Schellenberg B., Wagner U., Pauly M., Reiter W.-D., Ringli C. (2006). The Arabidopsis root hair cell wall formation mutant lrx1 is suppressed by mutations in the RHM1 gene encoding a UDP-l-rhamnose synthase. Plant Cell 18: 1630–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblin M.S., Pettolino F.A., Wilson S.M., Campbell R., Burton R.A., Fincher G.B., Newbigin E., Bacic A. (2009). A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-D-glucan synthesis in transgenic Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 5996–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe B.S., Kang B.-H., Gerl M.J., Gergely Z.R., McMichael C.M., Bednarek S.Y., Staehelin L.A. (2013). Cis-Golgi cisternal assembly and biosynthetic activation occur sequentially in plants and algae. Traffic 14: 551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G., van de Ven W., Pan S., Miao Y., Wang J., Keinath N.F., Weatherly B., Jiang L., Schumacher K., Hicks G., Raikhel N. (2012). Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res. 22: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois M., Gilles K.A., Hamilton J.K., Rebers P.T., Smith F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 8: 350–356. [Google Scholar]
- Dugard C.K., Mertz R.A., Rayon C., Mercadante D., Hart C., Benatti M.R., Olek A.T., SanMiguel P.J., Cooper B.R., Reiter W.D., McCann M.C., Carpita N.C. (2016). The cell wall arabinose-deficient Arabidopsis thaliana mutant murus5 encodes a defective allele of Reversibly Glycosylated Polypeptide2. Plant Physiol. 171: 1905–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley T.P.J., et al. (2006). Mapping the Arabidopsis organelle proteome. Proc. Natl. Acad. Sci. USA 103: 6518–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley T.P.J., Watson R., Griffin J.L., Dupree P., Lilley K.S. (2004). Localization of organelle proteins by isotope tagging (LOPIT). Mol. Cell. Proteomics 3: 1128–1134. [DOI] [PubMed] [Google Scholar]
- Ford K.L., Chin T., Srivastava V., Zeng W., Doblin M.S., Bulone V., Bacic A. (2016). Comparative ‘Golgi’ proteome study of Lolium multiflorum and Populus trichocarpa. Proteomes 4: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaut B.S., Doebley J.F. (1997). DNA sequence evidence for the segmental allotetraploid origin of maize. Proc. Natl. Acad. Sci. USA 94: 6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre D., Oh J., Boutté Y., Best J.G., Samuels L., Nilsson R., Uemura T., Marchant A., Bennett M.J., Grebe M., Bhalerao R.P. (2011). Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc. Natl. Acad. Sci. USA 108: 8048–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendre D., McFarlane H.E., Johnson E., Mouille G., Sjödin A., Oh J., Levesque-Tremblay G., Watanabe Y., Samuels L., Bhalerao R.P. (2013). Trans-Golgi network localized ECHIDNA/Ypt interacting protein complex is required for the secretion of cell wall polysaccharides in Arabidopsis. Plant Cell 25: 2633–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut D.M., Carpita N.C. (1990). Separation of membranes by flotation centrifugation for in vitro synthesis of plant cell wall polysaccharides. Protoplasma 156: 82–93. [Google Scholar]
- Gibeaut D.M., Carpita N.C. (1991). Tracing cell wall biogenesis in intact cells and plants: Selective turnover and alteration of soluble and cell wall polysaccharides in grasses. Plant Physiol. 97: 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut D.M., Carpita N.C. (1993). Synthesis of (1→3),(1→4)-β-d-glucan in the Golgi apparatus of maize coleoptiles. Proc. Natl. Acad. Sci. USA 90: 3850–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut D.M., Carpita N.C. (1994). Improved recovery of (1→3), (1→4)-β-d-glucan synthase activity from Golgi apparatus of Zea mays (L.) using differential flotation centrifugation. Protoplasma 180: 92–97. [Google Scholar]
- Groen A.J., Sancho-Andrés G., Breckels L.M., Gatto L., Aniento F., Lilley K.S. (2014). Identification of trans-Golgi network proteins in Arabidopsis thaliana root tissue. J. Proteome Res. 13: 763–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R., Lindeboom J.J., Paredez A.R., Emons A.M., Ehrhardt D.W. (2009). Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 11: 797–806. [DOI] [PubMed] [Google Scholar]
- Haigler C.H., Brown R.M. Jr (1986). Transport of rosettes from the Golgi apparatus to the plasma membrane in isolated mesophyll cells of Zinnia elegans during differentiation to tracheary elements in suspension culture. Protoplasma 134: 111–120. [Google Scholar]
- Heazlewood J.L., Verboom R.E., Tonti-Filippini J., Small I., Millar A.H. (2007). SUBA: The Arabidopsis Subcellular Database. Nucleic Acids Res. 35: D213–D218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé C., Duruflé H., San Clemente H., Albenne C., Balliau T., Zivy M., Dunand C., Jamet E. (2016). An enlarged cell wall proteome of Arabidopsis thaliana rosettes. Proteomics 16: 3183–3187. [DOI] [PubMed] [Google Scholar]
- Kang B.-H., Nielsen E., Preuss M.L., Mastronarde D., Staehelin L.A. (2011). Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329. [DOI] [PubMed] [Google Scholar]
- Kim J.-B., Carpita N.C. (1992). Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiol. 98: 646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-J., Zemelis S., Keegstra K., Brandizzi F. (2015). The cytoplasmic localization of the catalytic site of CSLF6 supports a channeling model for the biosynthesis of mixed-linkage glucan. Plant J. 81: 537–547. [DOI] [PubMed] [Google Scholar]
- Kong Y., et al. (2015). Galactose-depleted xyloglucan is dysfunctional and leads to dwarfism in Arabidopsis. Plant Physiol. 167: 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrador E., Nicolás G. (1984). Autolysis of cell walls in pea epicotyls during growth. Enzymatic activities involved. Physiol. Plant. 64: 541–546. [Google Scholar]
- Langmead B., Salzberg S.L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9: 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lending C.R., Lippman E., Bracker C.E., Bartnicki-Garcia S. (1990). An efficient preparative isopycnic flotation method for the isolation of chitosomes. Protoplasma 159: 16–25. [Google Scholar]
- Liu L., Shang-Guan K., Zhang B., Liu X., Yan M., Zhang L., Shi Y., Zhang M., Qian Q., Li J., Zhou Y. (2013). Brittle Culm1, a COBRA-like protein, functions in cellulose assembly through binding cellulose microfibrils. PLoS Genet. 9: e1003704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Paulitz J., Pauly M. (2015). The presence of fucogalactoxyloglucan and its synthesis in rice indicates conserved functional importance in plants. Plant Physiol. 168: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madson M., Dunand C., Li X., Verma R., Vanzin G.F., Caplan J., Shoue D.A., Carpita N.C., Reiter W.D. (2003). The MUR3 gene of Arabidopsis encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. Plant Cell 15: 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann M.C., Defernez M., Urbanowicz B.R., Tewari J.C., Langewisch T., Olek A., Wells B., Wilson R.H., Carpita N.C. (2007). Neural network analyses of infrared spectra for classifying cell wall architectures. Plant Physiol. 143: 1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz R.A., Olek A.T., Carpita N.C. (2012). Alterations in cell-wall glycosyl linkage structure of Arabidopsis murus mutants. Carbohydr. Polym. 89: 331–339. [DOI] [PubMed] [Google Scholar]
- Nebenführ A., Staehelin L.A. (2001). Mobile factories: Golgi dynamics in plant cells. Trends Plant Sci. 6: 160–167. [DOI] [PubMed] [Google Scholar]
- Nikolovski N., Rubtsov D., Segura M.P., Miles G.P., Stevens T.J., Dunkley T.P.J., Munro S., Lilley K.S., Dupree P. (2012). Putative glycosyltransferases and other plant Golgi apparatus proteins are revealed by LOPIT proteomics. Plant Physiol. 160: 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolovski N., Shliaha P.V., Gatto L., Dupree P., Lilley K.S. (2014). Label-free protein quantification for plant Golgi protein localization and abundance. Plant Physiol. 166: 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons H.T., et al. (2012). Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol. 159: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons H.T., Drakakaki G., Heazlewood J.L. (2013). Proteomic dissection of the Arabidopsis Golgi and trans-Golgi network. Front. Plant Sci. 3: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons H.T., González Fernández-Niño S.M., Heazlewood J.L. (2014). Separation of the plant Golgi apparatus and endoplasmic reticulum by free-flow electrophoresis. In Jorrin-Novo J.V., Komatsu S., Weckwerth W., Wienkoop S., eds, Plant Proteomics: Methods and Protocols, Methods in Molecular Biology, Vol. 1072 Springer Science and Business Media, New York, pp. 527–539. [DOI] [PubMed] [Google Scholar]
- Pattathil S., et al. (2010). A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153: 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S., Avci U., Miller J.S., Hahn M.G. (2012). Immunological approaches to plant cell wall and biomass characterization: Glycome profiling. Methods Mol. Biol. 908: 61–72. [DOI] [PubMed] [Google Scholar]
- Pattathil S., Avci U., Zhang T., Cardenas C.L., Hahn M.G. (2015). Immunological approaches to biomass characterization and utilization. Front. Bioeng. Biotechnol. 3: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M.J., Ryden P., Madson M., Smith A., Reiter W.-D., Carpita N.C. (2004). The galactose residues of xyloglucan are essential to maintain mechanical strength of the primary cell walls in Arabidopsis during growth. Plant Physiol. 134: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning B.W., et al. (2009). Genetic resources for maize cell wall biology. Plant Physiol. 151: 1703–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning B.W., et al. (2014). Genetic determinants for enzymatic digestion of lignocellulosic biomass are independent of those for lignin abundance in a maize recombinant inbred population. Plant Physiol. 165: 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y., et al. (2019). The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 47 (D1): D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhlmann J., Bucheli E., Swain M.J., Dunning N., Albersheim P., Darvill A.G., Hahn M.G. (1994). Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal α-(1→2)-linked fucosyl-containing epitope. Plant Physiol. 104: 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W.D., Vanzin G.F. (2001). Molecular genetics of nucleotide sugar interconversion pathways in plants. Plant Mol. Biol. 47: 95–113. [PubMed] [Google Scholar]
- Reiter W.-D., Chapple C.C.S., Somerville C.R. (1993). Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science 261: 1032–1035. [DOI] [PubMed] [Google Scholar]
- Rosquete M.R., Davis D.J., Drakakaki G. (2018). The plant trans-Golgi network: Not just a matter of distinction. Plant Physiol. 176: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht C., Bartetzko M.P., Senf D., Dallabernadina P., Boos I., Andersen M.C.F., Kotake T., Knox J.P., Hahn M.G., Clausen M.H., Pfrengle F. (2017). A synthetic glycan microarray enables epitope mapping of plant cell wall glycan-directed antibodies. Plant Physiol. 175: 1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden P., Sugimoto-Shirasu K., Smith A.C., Findlay K., Reiter W.-D., McCann M.C. (2003). Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 132: 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffer A.M., Carpita N.C., Irish V.F. (2017). Rhamnose-containing cell wall polymers suppress helical plant growth independently of microtubule orientation. Curr. Biol. 27: 2248–2259.e4. [DOI] [PubMed] [Google Scholar]
- Scheller H.V., Ulvskov P. (2010). Hemicelluloses. Annu. Rev. Plant Biol. 61: 263–289. [DOI] [PubMed] [Google Scholar]
- Schmidt D., Schuhmacher F., Geissner A., Seeberger P.H., Pfrengle F. (2015). Automated synthesis of arabinoxylan-oligosaccharides enables characterization of antibodies that recognize plant cell wall glycans. Chemistry 21: 5709–5713. [DOI] [PubMed] [Google Scholar]
- Shedletzky E., Shmuel M., Delmer D.P., Lamport D.T.A. (1990). Adaptation and growth of tomato cells on the herbicide 2,6-dichlorobenzonitrile leads to production of unique cell walls virtually lacking a cellulose-xyloglucan network. Plant Physiol. 94: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedletzky E., Shmuel M., Trainin T., Kalman S., Delmer D. (1992). Cell wall structure in cells adapted to growth on the cellulose-synthesis inhibitor 2,6-dichlorobenzonitrile: A comparison between two dicotyledonous plants and a gramineous monocot. Plant Physiol. 100: 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A.M. (1993). Structure and function of plant cell wall proteins. Plant Cell 5: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan W., Kovác P., Albersheim P., Darvill A.G., Hahn M.G. (1995). Characterization of a monoclonal antibody that recognizes an arabinosylated (1→6)-β-d-galactan epitope in plant complex carbohydrates. Carbohydr. Res. 275: 295–307. [DOI] [PubMed] [Google Scholar]
- Sun Q., Zybailov B., Majeran W., Friso G., Olinares P.D., van Wijk K.J. (2009). PPDB, the plant proteomics database at Cornell. Nucleic Acids Res. 37: D969–D974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., et al. (2013). An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25: 270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Showalter A.M., Egelund J., Hernandez-Sanchez A., Doblin M.S., Bacic A. (2012). Arabinogalactan-proteins and the research challenges for these enigmatic plant cell surface proteoglycans. Front. Plant Sci. 3: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N.G. (2008). Cellulose biosynthesis and deposition in higher plants. New Phytol. 178: 239–252. [DOI] [PubMed] [Google Scholar]
- Terry M.E., Bonner B.A. (1980). An examination of centrifugation as a method of extracting an extracellular solution from peas, and its use for the study of indoleacetic acid-induced growth. Plant Physiol. 66: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry M.E., Jones R.L. (1981). Soluble cell wall polysaccharides released from pea stems by centrifugation: I. Effect of auxin. Plant Physiol. 68: 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K., Goto Y., Asatsuma S., Koizumi M., Mitsui T., Matsuoka K. (2009). A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 21: 1212–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Suda Y., Ueda T., Nakano A. (2014). Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol. 55: 694–703. [DOI] [PubMed] [Google Scholar]
- Urbanowicz B.R., Rayon C., Carpita N.C. (2004). Topology of the maize mixed-linkage (1→3),(1→4)-β-d-glucan synthase at the Golgi membrane. Plant Physiol. 134: 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vain T., Crowell E.F., Timpano H., Biot E., Desprez T., Mansoori N., Trindade L.M., Pagant S., Robert S., Höfte H., Gonneau M., Vernhettes S. (2014). The cellulase KORRIGAN is part of the cellulose synthase complex. Plant Physiol. 165: 1521–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzin G.F., Madson M., Carpita N.C., Raikhel N.V., Keegstra K., Reiter W.D. (2002). The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proc. Natl. Acad. Sci. USA 99: 3340–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Chen X., Goldbeck C., Chung E., Kang B.H. (2017). A distinct class of vesicles derived from the trans-Golgi mediates secretion of xylogalacturonan in the root border cell. Plant J. 92: 596–610. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Ho Y.Y., Lampugnani E.R., Van de Meene A.M.L., Bain M.P., Bacic A., Doblin M.S. (2015). Determining the subcellular location of synthesis and assembly of the cell wall polysaccharide (1,3; 1,4)-β-d-glucan in grasses. Plant Cell 27: 754–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Wang M., Shi D., Zhou G., Niu T., Hahn M.G., O’Neill M.A., Kong Y. (2017). DGE-Seq analysis of MUR3-related Arabidopsis mutants provides insight into how dysfunctional xyloglucan affects cell elongation. Plant Sci. 258: 156–169. [DOI] [PubMed] [Google Scholar]
- Zablackis E., Huang J., Müller B., Darvill A.G., Albersheim P. (1995). Characterization of the cell-wall polysaccharides of Arabidopsis thaliana leaves. Plant Physiol. 107: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabotina O.A., Avci U., Cavalier D., Pattathil S., Chou Y.-H., Eberhard S., Danhof L., Keegstra K., Hahn M.G. (2012). Mutations in multiple XXT genes of Arabidopsis reveal the complexity of xyloglucan biosynthesis. Plant Physiol. 159: 1367–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., et al. (2016). Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis. Nat. Commun. 7: 11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.F., Staehelin L.A. (1992). Functional compartmentation of the Golgi apparatus of plant cells: Immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 99: 1070–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]