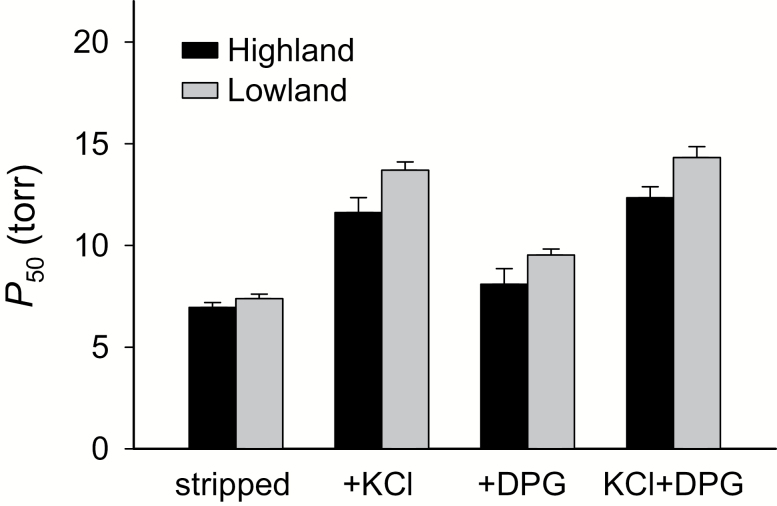
Fig. 3.
Highland deer mice (Peromyscus maniculatus) from the Rocky Mountains have a higher Hb-O2 affinity than lowland mice from the Great Plains, as indicated by lower values of P50 (the PO2 at which Hb is 50% saturated). O2 equilibria were measured at pH 7.40, 37°C, in the presence and absence of allosteric cofactors that modulate Hb-O2 binding ([Cl−], 0.10 mol/l; [HEPES], 0.1 mol/l; DPG:tetrameric Hb ratio, 2.0: [heme], 0.2–0.3 mmol/l). P50 values (± SE) are reported for stripped Hbs in the absence of added anions, in the presence of Cl− alone (added as KCl), in the presence of DPG alone, and in the presence of both anions combined. This latter “KCl+DPG” treatment is most relevant to in vivo conditions in mammalian red blood cells, but measurements of O2 affinity under each of the four treatments provide insights into the functional mechanism responsible for observed differences in P50(KCl+DPG). Data from Natarajan et al. (2015a) (for additional details, see Storz et al. 2009, 2010a and Jensen et al. 2016).