Figure 2: Michaelis-Menten data points fitted to the mixed inhibition rate equation and Double reciprocal plots.
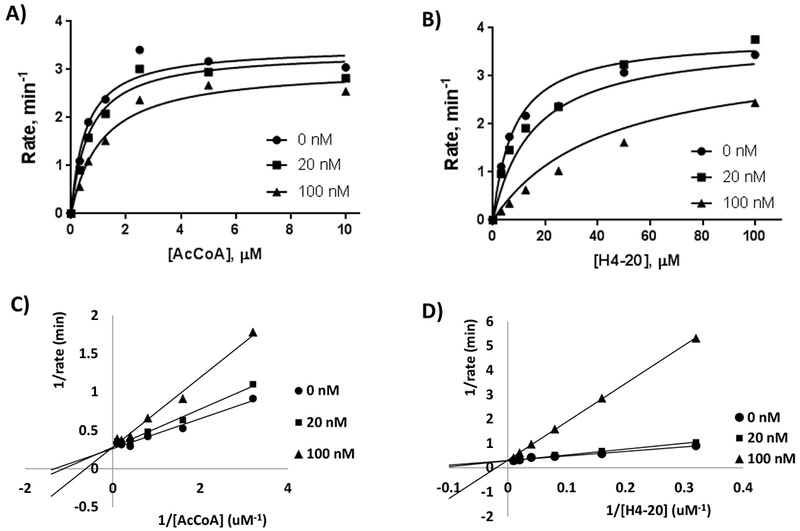
To determine the mode of inhibition in respect to AcCoA and H4-20 peptide, H4K12CoA was added to the reaction mixture at 0 nM, 20 nM, and 100 nM. A) Conditions to determine HAT1 kinetics in respect to AcCoA included 100 µM H4-20 peptide, 5 nM HAT1, and 0-10 µM AcCoA, with a reaction time of 9 min. B) Conditions to determine HAT1 kinetics in respect to H4-20 peptide included 3 µM AcCoA, 20 nM HAT1, and 0-100 µM AcCoA, with a reaction time of 9 min. Double reciprocal plots of H4K12CoA with respect to AcCoA (C) and H4-20 (D).
