Abstract
This study examines interactions of heritable influences, prenatal substance use, and postnatal parental warmth and hostility on the development of conduct problems in middle childhood for boys and girls. Participants are 561 linked families, collected in two cohorts, including birth parents, adoptive parents, and adopted children. Heritable influences on internalizing and externalizing (including substance use) problems were derived from birth mothers’ and fathers’ symptoms, diagnoses, and age of onset from diagnostic interviews, and the proportion of first-degree relatives with the same type of problems. Smoking during pregnancy (SDP) and alcohol use during pregnancy were assessed retrospectively from birth mothers at 5 months post-partum. Earlier externalizing problems and parental warmth and hostility and were assessed at one assessment prior to the outcome (Cohort II: 4.5 years; Cohort I: 7 years). Conduct problems were symptoms from a diagnostic interview assessed at age 6 (Cohort II) or 8 (Cohort I). Findings from regression analyses suggest that 1) SDP plays an important role for the development of conduct problems, 2) some relatively well-accepted effects (e.g., parental hostility) were less important when simultaneously considering multiple factors influencing the development of conduct problems, and 3) main effects of genetic risk and SDP, and interactions among genetic risk and postnatal warmth, SDP and postnatal warmth, and genetic risk, SDP and postnatal hostility for conduct problems were important for boys’ but not girls’ conduct problems. Replication is needed, but the current results provide preliminary but empirically-grounded hypotheses for future research testing complex developmental models of conduct problems.
Keywords: genetic, smoking during pregnancy, alcohol use during pregnancy, parent-child relationship, conduct problems, adoption
The role of genetic and environmental influences on the development of conduct problems has been the focus of a large body of research. Thus far, it is clear that a) there is a genetic component underlying conduct problems, b) both prenatal and postnatal environments play important roles in the development of conduct problems, and c) genetic influences interact with several environmental influences in such a way that increases risk for conduct problems (e.g., see Holz et al., 2016 for review). Despite elegant and comprehensive theoretical frameworks highlighting the multi-level, interactive role of genetic (and subsequent biological development) and environmental influences on the development of conduct problems, as well as studies examining gene-environment interactions using a variety of different environmental influences (e.g., Dodge, 2009; Holz et al., 2016), studies have generally not examined more complex models including interactions of genetic influences and multiple environmental influences unfolding over time for the development of conduct problems. The focus of this study is to assess how genetic, prenatal, and postnatal environmental influences interact in helping to shape the development of conduct problems in boys and girls in middle childhood.
Parenting Influences on Child Conduct Problems
Parent-to-child transmission explains a substantial portion of individual differences in conduct problems in middle childhood (see Burt, 2009b; and Salvatore & Dick, 2016 for a recent review of genetic influences, and the role of gene-environment interplay on conduct problems). Parental harshness or negativity has emerged as a key specific environmental influence important for conduct problems (O'Connor, Deater-Deckard, Fulker, Rutter, & Plomin, 1998; Pajer et al., 2008). Indeed, when examined within genetically informed designs, parental negativity (Pike, McGuire, Hetherington, & Reiss, 1996) and parent-child conflict (Burt, Krueger, McGue, & Iacono, 2003; Burt, McGue, Krueger, & Iacono, 2007; Klahr, McGue, Iacono, & Burt, 2011), have accounted for substantial proportions (e.g., ~15–25%) of the total shared environmental variance in externalizing problems, including conduct problems, suggesting that parenting influences in middle childhood can exert an influence even after accounting for genetic influences or gene-environment correlation.
Early socialization models have highlighted the important role of parents for shaping children’s development, although pure socialization theories have gained less prominence in favor of theories of reciprocal interaction (Shaw & Bell, 1993; Stice & Barrera, 1995). The theory of coercive cycles illustrates how parental negativity, hostility, and harshness can influence the development of conduct problems (e.g., Patterson, Reid, & Dishion, 1998), highlighting that while parents’ harsh discipline is often a response to children’s poor behavior, the harsh discipline itself contributes to increased conduct problems in youth, and this process canalizes over time. Indeed, parenting interventions are among the most commonly used to reduce early and middle childhood conduct problems, often focusing on teaching parents to replace negative behaviors that feed into coercive cycles with positive parenting behaviors (e.g., warmth, positive contingent responses) shown to be protective against conduct problems (Shelleby & Shaw, 2014). These types of interventions generally show effectiveness, particularly for children who showed elevated conduct problems earlier in development (Shelleby & Shaw, 2014). Thus, considering theory and evidence from genetically informed and intervention science, the literature suggests that parenting is important as a unique environmental influence in middle childhood.
Prenatal Influences on Child Conduct Problems
The prenatal environment has also been identified as an important influence on the development of conduct problems in children. Although an array of prenatal environmental influences has been associated with conduct problems in children (e.g., obstetric complications, prenatal stress and internalizing problems, Gaysina et al., 2013; Glover, 2011; Lukkari et al., 2012; O’Connor, Heron, Golding, Beveridge, & Glover, 2002), the strongest evidence for the role of prenatal risk on children’s conduct problems is maternal substance use during pregnancy. For example, exposure to maternal smoking during pregnancy (SDP) has been associated with an increased risk of conduct problems from childhood into adulthood (Langley, Holmans, van den Bree, & Thapar, 2007; Nigg & Breslau, 2007; Wakschlag & Hans, 2002; Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2002). However, there is still debate as to whether the prenatal environment, and specifically exposure to substance use during pregnancy, is linked to conduct problems via biological or environmental mechanisms (as opposed to genetic or familial confounding, Knopik, 2009; Paradis, Shenassa, Papandonatos, Rogers, & Buka, 2017). There is substantial work in animal models investigating prenatal exposure to nicotine that shows convincing biological pathways by which prenatal exposure to specific drugs (e.g., nicotine) can alter brain development in such a way that may put animals at risk for altered behavioral patterns (Slikker Jr, Xu, Levin, & Slotkin, 2005; Slotkin, 1992). There is also some corroborating evidence in humans (Bublitz & Stroud, 2012; Ekblad, Korkeila, & Lehtonen, 2015) that similar biological pathways may be implicated in how exposure to maternal drug use during pregnancy may directly affect brain and behavioral development in humans (see Ernst, Moolchan, & Robinson, 2001; Knopik, 2009 for reviews).
Most of the recent studies using genetically sensitive designs like sibling-comparison approaches and in-vitro fertilization have found that the association between SDP and conduct problems arises because of confounding variables common to parents and children, suggesting that SDP is not a direct environmental influence leading to increased conduct problems (Ellingson, Goodnight, Van Hulle, Waldman, & D’Onofrio, 2014; Ellingson, Rickert, Lichtenstein, Langstrom, & D’Onofrio, 2012; Kuja-Halkola, D’Onofrio, Larsson, & Lichtenstein, 2014; Rice et al., 2009; but see Gaysina et al., 2013 for the opposite conclusion). Prenatal exposure to substance use, in this case SDP, therefore could mark an inherited risk rather than be a unique environmental risk (Marceau et al., 2016; Rice et al., 2009), highlighting the likely role of passive gene-environment correlation (rGE) in this association (D’Onofrio et al., 2003; Knopik et al., 2006; Maughan, Taylor, Caspi, & Moffit, 2004). Although SDP is arguably the best studied prenatal influence on conduct problems thus far, other drugs have also been implicated in the development of conduct problems in childhood: most notably exposure to alcohol use during pregnancy (ADP; Burd, Klug, Martsolf, & Kerbeshian, 2003; D’Onofrio et al., 2007; Fryer, McGee, Matt, Riley, & Mattson, 2007; Steinhausen & Spohr, 1998; Ware et al., 2013).
Genotype-Environment Interaction
Genes and environments do not operate in isolation, but rather the environment can moderate the effects of genetic influences and vice versa (genotype-environment interaction, G × E). G × E has been shown to be a key mechanism by which genetic and environmental influences are jointly associated with conduct problems (Holz et al., 2016). Most of the G × E work on conduct problems has focused on postnatal environmental influences. Twin studies in adolescence have shown that genetic influences are typically stronger in contexts of environmental adversity (e.g., parental negativity; Button, Lau, Maughan, & Eley, 2008; Feinberg, Button, Neiderhiser, Reiss, & Hetherington, 2007) or restrictive environments (Salvatore & Dick, 2016), consistent with a diathesis-stress framework, or social opportunity or social control mechanisms of G × E (e.g., that genetic effects are dampened by social control but elevated when individuals are in environments that support genetic expression; Shanahan & Hofer, 2005).
However, across several genetically informed studies of conduct problems in twin children (as opposed to adolescents), G × E findings were more consistent with a ‘social push’ mechanism (Burt & Klump, 2014). That is, adverse experiences (peer deviance, parent-child conflict, and disadvantaged neighborhoods) are such a strong influence on the development of conduct problems that they overwhelm genetic influence. Thus, genes are only able to exert a meaningful influence on conduct problems (e.g., account for a larger proportion of the variance in conduct problems) in the presence of low adverse environmental conditions (Burt & Klump, 2013, 2014; Burt, Klump, Gorman-Smith, & Neiderhiser, 2016). In a sample of 6–10 year old twins, Burt and colleagues (2003) showed that maternal warmth and directiveness jointly moderated genetic influences on conduct problems. Shared environmental influences played a larger role in the etiology of conduct problems for children who experienced less directive and ‘colder’ parenting by mothers, whereas genetic influences were stronger among children experiencing warmer and more directive mothering. Notably, these social opportunity/control and social push mechanisms describe how different parenting contexts affect the magnitude of genetic and environmental influences on conduct problems and are useful for understanding the etiology of conduct problems in given contexts. Although these mechanisms and findings are important for understanding G × E for conduct problems in middle childhood, they are specific to twin studies since twin studies estimate a latent genetic influence, rather than a directional, specified genetic or heritable risk factor as employed in adoption and measured gene studies. Most studies examining measured, directional heritable risk by postnatal environmental influences on conduct problems, as in the present study, are consistent with diathesis-stress models in across multiple developmental stages (Cadoret, Cain, & Crowe, 1983; Ge et al., 1996; Leve et al., 2010).
The joint role of genetic influences and prenatal exposure to maternal substance use is less frequently studied. Candidate gene × SDP studies suggest that some children are genetically predisposed to be more sensitive to the effects of cigarette smoke (P. A. Brennan et al., 2011; Kahn, Khoury, Nichols, & Lanphear, 2003; Wakschlag et al., 2009), and generally suggest that SDP (and potentially other exposures, e.g., ADP) allows for genetic influences on externalizing problems to be more highly influential, although this pattern was not always found and the “risk” allele sometimes differs across studies (e.g., Becker, El-Faddagh, Schmidt, Esser, & Laucht, 2008; Hohmann et al., 2016; Kahn et al., 2003; Salatino-Oliveira et al., 2016; Wakschlag et al., 2009).
Prenatal x Postnatal Environment Interactions
There is also very limited evidence of prenatal × postnatal environment interactions in the development of externalizing problems, most frequently examined in biological families where the prenatal and postnatal environments are confounded with each other (e.g., mothers who used substances prenatally and continue postnatally) and with genetic influences. Despite those limitations, maternal negativity exacerbated the effects of prenatal cocaine exposure for externalizing problems (Molnar, Levitt, Eiden, & Schuetze, 2014). In all, there is evidence of multiple important environmental influences and interactions among those influences on conduct problems, but few studies have considered multiple moderators of genetic or environmental influences on conduct problems (see Burt, Klahr, Neale, & Klump, 2013; Lipscomb et al., 2014 for exceptions). Theoretically, the broader contextual milieu is likely to be important for conduct problems. Indeed, both prior studies examining three-way interactions of genetic and multiple environmental influences support that the combined risks amplify effects of each influence to put children at the very highest levels of risk for developing conduct problems. Corroborating this pattern for a related phenotype, a third study that used data from the current sample examined genetic x prenatal x postnatal environment interactions on directionality, a measure of whether children exhibit more externalizing relative to internalizing symptoms or vice versa. Findings suggested that the combination higher genetic risk for externalizing problems, more pregnancy complications, and higher marital hostility was associated with externalizing symptoms in 4.5-year-old children. In contrast, when genetic risk for externalizing problems was low, pregnancy complications were associated with internalizing symptoms in 4.5-year-old children at any level of marital hostility, and when marital hostility was low, there was no effect of pregnancy complications on directionality of symptoms (Neiderhiser et al., 2016). In all, there is evidence that considering multiple moderators is important for the development of conduct problems and related phenotypes (e.g., externalizing problems earlier in development).
Sex Differences
Sex differences in conduct problems have been documented beginning around age 4 (Brennan & Shaw, 2013; Burt et al., 2013), and although no sex differences in the magnitude of genetic influences on conduct problems were found in a meta-analysis (Burt, 2009), generally sex differences in G × E findings have not been tested (Burt et al., 2013; Burt & Klump, 2014). A notable exception is the body of studies examining MAOA genotype – environment interactions for conduct problems. A recent meta-analysis showed the opposite effect of MAOA variation in combination with different environmental influences for boys’ and girls’ antisocial behavior such that the low-activity MAOA genotype interacted with a general, composite early adversity to predict antisocial behavior in males, but only the high-activity allele interacted specifically with a history of child maltreatment to predict antisocial behavior in females (age range not specified; Byrd & Manuck, 2014). This may be a special case, however, since MAOA is located on the X chromosome and thus there are gender differences in the number of MAOA alleles present in males and females.
There is somewhat more evidence of sex differences in prenatal influences (relative to heritable influences) on behavior (Molnar et al., 2014; Weinstock, 2007), though not ubiquitous (Maughan et al., 2004; Rice et al., 2009). Although infrequently tested, sex differences in gene × prenatal interactions for conduct problems have been found (Hohmann et al., 2016). In the absence of evidence from twin and adoption studies, we briefly summarize findings using a candidate gene approach. For example, Becker et al. (2008) ENREF_3 found that the interaction of DAT1 and SDP was associated with conduct problems only in boys, not girls. Further, Wakschlag et al. (2009) showed the low-activity MAOA genotype interacted with SDP in boys to predict conduct problems, whereas the high-activity allele interacted with SDP in girls to predict conduct problems. These findings mirror the sex-specific G × E (early adversity) findings with MAOA reviewed in (Byrd & Manuck, 2014).
In addition to sex differences in rates of conduct problems and potential sex differences in G × E literature, there is evidence that boys are more vulnerable to variations in the early environment than girls. This pattern is evidenced by higher rates of miscarriage, stillbirth, and death in the first year, and higher rates of neuropsychological disorders early in childhood (Kraemer, 2000). Further, boys may be more influenced by the early parenting environment: low maternal responsivity at one year has been associated with boys’ but not girls’ conduct problems in early childhood (e.g., Shaw, Keenan, & Vondra, 1994). Based on these findings, we included child sex as a potential moderator of genetic × prenatal × postnatal environmental interactions in the present study. Specifically, we would expect that genetic × prenatal × postnatal environmental interactions may be more influential for boys, as they may be more vulnerable to the exacerbating effects of multiple genetic and environmental risk factors than girls.
Present Study
The present study is among the first to examine multiple key environmental influences associated with the development of conduct problems in interaction with genetic influences, and to disentangle genetic from prenatal from postnatal influences for childhood conduct problems. Specifically, we tested for interactions among inherited influences, prenatal exposures (SDP and ADP), and parental warmth and hostility for the development of conduct problems during middle childhood. Because conduct problems are generally stable and increasing across childhood, and because trajectories of conduct problems are associated with other externalizing behaviors (Van Lier, Der Ende, Koot, & Verhulst, 2007), we also controlled for earlier externalizing problems in order to assess development of conduct problems.
We used a unique study design of children adopted at birth that allowed us to examine the associations between (a) children with biological parents (who provide genetic influences [and prenatal influences in the case of the birth mother] but not postnatal environmental influences) and (b) children with adoptive parents (who provide postnatal influences, but not genetic or prenatal environmental influences; Leve et al., 2013). This study design takes advantage of a natural experiment separates genetic (and prenatal) influences from postnatal environmental influences, improving on prior studies examining prenatal × postnatal interactions in biologically-related families where the prenatal and postnatal environment (and genetic material) are provided by the same individuals. We also used both birth mother and birth father information to inform our indices of heritable risk for psychopathology and paid careful attention to the timing of psychopathology symptoms and disorders in birth mothers to more cleanly separate genetic from prenatal environmental influences than has been previously available in studies of this nature. The adoption design uses birth parent characteristics as indicators of genetic risk, which has the advantage of being broader and more likely to capture polygenic effects in heritable risk than candidate gene studies, but also has a more specified definition of genetic risk (e.g., the birth parent characteristic measured) than twin studies, in which estimated genetic influences are entirely latent. Thus, this adoption design is well-suited to bridge G × E findings from candidate gene and twin studies, adding an important perspective on G × E findings to the literature.
Of note, findings from intergenerational studies and earlier findings from this adoption sample have shown that internalizing problems (including anxiety and depressive phenotypes) in the biological parent generation are also associated with child externalizing phenotypes (Kim et al., 2009), at least in part through genetic mechanisms (e.g., in toddlerhood: Pemberton et al., 2010; Marceau et al., 2013; Kerr et al., 2013; in adolescence: Silberg et al., 2011; Singh et al., 2011). The reverse has also been found: substance use disorder in parent and grandparent generations has been associated with emotional disorders in children (Leventhal, Pettit, Lewinsohn, 2011). Evidence from twin studies suggest that some of the same genetic influences contribute to both child internalizing and externalizing problems (e.g., in adolescence: Cosgrove 2011; Lahey et al., 2011; O’Connor et al., 1998). Even conduct problems more specifically share some common genetic influences with depression (Subbarao et al., 2008). Taken together, genetic influences on conduct problems in childhood may be indexed through birth parent externalizing or internalizing psychopathologies, and thus we include genetic influence indicators for both.
Our hypotheses were drawn from several literatures. Based on findings of gene × parenting interactions in childhood, we expected parenting to moderate heritable influences such that heritable risk for conduct problems would be present in the context of high parental hostility and low parental warmth but absent in conditions of average and low parental hostility and average and high parental warmth, consistent with a diathesis stress mechanism. Based on findings of gene × prenatal influences, we expected that prenatal exposure to SDP or ADP, would exacerbate heritable risk for conduct problems in children. We expected three-way interactions of heritable, prenatal, and parenting influences such that low parental warmth and high parental hostility would exacerbate the two-way gene × prenatal interactions, building on studies suggesting that parental hostility exacerbates the effects of the prenatal environment (which exacerbates the effects of heritable risk). Finally, we also examined sex differences in these hypotheses as an exploratory analysis, given that sex differences in genetic × prenatal and genetic × postnatal environment interactions on conduct problems have sometimes been found, though infrequently tested.
Methods
Participants
Participants included 561 linked families including birth parents (BP), adoptive parents (AP), and adopted children from the Early Growth and Development Study (EGDS, described in detail in Leve et al., 2013). The study was approved by the Institutional Review Board at the University of Oregon (The Early Growth and Development Study Pediatric Cohort (EGDS-ECHO), Protocol # 08082016.007; IRB of record) and at Purdue University (Early Growth and Development Study, Protocol # 1701018706), as well as other participating Institutions. Data from these participants were collected in two cohorts (Cohort I recruited from 2003–2006; Cohort II recruited from 2008–2010). Recruitment was facilitated by 45 adoption agencies in 15 states reflecting the full range of adoption agencies in the US (e.g., public vs. private, religious vs. secular, favoring more open vs. more closed adoptions). Families were eligible if the adoption was domestic, placement occurred within 3 months postpartum, the child was placed with a non-relative, the child had no major medical conditions, and BP and APs could understand English at the 8th grade level.
Data were collected in-home and via web-based questionnaires and phone interviews beginning 3–6 months post-partum and extending across infancy through middle childhood (and further assessments are ongoing), assessing a wide range of BP and AP characteristics, prenatal and postnatal environments, and child behavioral and cognitive outcomes. The current study includes BP (indexing genetic influences), prenatal, postnatal parenting, and child outcome data. Data on prenatal influences are drawn from assessments from the birth mother (BM) when children were approximately 5 months old (n = 556). Data on heritable influences were drawn from both BPs when children were approximately 18 months old (n = 514 BM, 188 birth fathers [BF]), both BPs when the children were approximately 56 months old in Cohort I only (n = 328 BM, 110 BF). AP assessment schedules varied for the two cohorts because of differences in funding sources and timelines. Outcome data were drawn from a recently completed assessment of APs, when children in Cohort II were 6 years old and in Cohort I were 8 years old (n = 403). We also measured externalizing problems from the assessment immediately preceding the outcome assessment, when children in Cohort II were 4.5 years and in Cohort I were 7 years old (n = 456), in order to examine the development of conduct problems. Data on postnatal parenting were drawn from the same proximal assessments of APs as were earlier externalizing problems, in order to examine the proximal role of parenting for the development of conduct problems (supported by the standard practice and adequacy of model fit for cross-lagged models of parenting and child conduct problems to consider single T+1 lags, without needing to fit longer lags, e.g., Pardini, Fite, & Burke, 2008; Shaffer, Lindhiem, Kolko, & Trentacosta, 2013).
The average placement age was 6 days post-partum (SD = 12.45, maximum = 91 days). Children were 57% male, most were White Non-Hispanic (55.6%), or multi-ethnic (19.3%), Black or African American (13%), or Hispanic (10.9%). Adoptive family compositions were mostly characterized by both an adoptive mother and father (90.8% at the start of the study, 85.5% at the wave of the parenting assessment included in this study), with some same-sex APs (7.0% at the start of the study, 6.6% at the parenting assessment), single APs (1.8% at the start of the study, 1.5% at the parenting assessment), or separated or divorced (none at the start of the study, 6% at the parenting assessment). Demographic characteristics for BPs and APs are presented in Table 1. BPs were more ethnically diverse than APs (e.g., ~70% White vs. ~90% White). BPs were less advantaged than APs at the beginning of the study, as indicated by being younger (relative to APs) at the time of placement (on average in the mid-20’s vs. the mid-late 30’s), having lower education (median education level of high school vs. 4-year college), and lower income (median income below $40,000 vs. between $100,000-$150,000).
Table 1.
Sample Descriptive Statistics
| Variable | Birth Mothers | Birth Fathers | Adoptive Parent 1 | Adoptive Parent 2 |
|---|---|---|---|---|
| Age at child birth | 24.35 (6.03) | 26.10 (7.78) | 37.4 (5.57) | 38.24 (5.85) |
| Race | ||||
| Caucasian | 70.1% | 69.9% | 91.8% | 90.4% |
| African-American | 13.3% | 11.5% | 3.9% | 4.9% |
| Hispanic/Latino | 6.7% | 9.6% | 2.0% | 1.6% |
| Multiethnic | 4.9% | 4.8% | 0.9% | 1.1% |
| Other | 5.0% | 4.2% | 1.4% | 1.0% |
| Median Education Level | High School | High School | 4-year college | 4-year college |
| Median Annual Income | $15,001–25,000 | $25,001–40,000 | $100,001 - $150,000 | |
| Employment | ||||
| Full Time | 38.2% | 54.3% | 38.9% | 76.9% |
| Part Time | 15.6% | 11.1% | 17.7% | 2.4% |
| Unemployed but looking for work | 17.0% | 15.4% | 0.9% | 1.3% |
| Full-time homemaker | 9.1% | 1.3% | 22.9% | 1.8% |
| Other | 20.1% | 17.9% | 19.6% | 17.6% |
| Marital Status | ||||
| Single, never married | 37.7% | 37.6% | 1.6% | 0.2% |
| Married | 22.2% | 27.1% | 84.6% | 86.5% |
| Living in a committed relationship | 30.1% | 31.0% | 2.7% | 1.6% |
| Other | 10% | 4.3% | 11.1% | 11.7% |
Missing Data.
There were two main sources of missing data in the current study: BF participation, and attrition. We examined differences in study variables and sample demographics across groups where (1) BFs did vs. did not participate, and (2) the outcome variable was missing vs. available (to index attrition) using a series of Kruskal-Wallace one-way analysis of variance tests (PROC NPAR1WAY, Wilcoxon using SAS statistical software). Variables investigated included conduct problems (for the analysis of BF missingness only), genetic risk variables (based on BM data for BF missingness, and based on BP and on only BM data for attrition), substance use frequency variables, parent-child warmth and hostility variables, and AP and BM age at child’s birth, AP household income, AP1 and AP2 education, adoption process variables (openness and knowledge, described below), child ethnicity, sex, and placement age (a total of 47 tests, adjusted p = .0011). BF participation was related to adoption process variables: families where BFs participated had higher knowledge scores, χ2(1) = 49.84, p < .0001, and were more open, χ2(1) = 10.75, p = .001. There were no other differences in study or demographic variables, χ2’s(1) < 3.58, p’s > .058. None of the study or demographic variables were related to attrition, χ2’s(1) < 4.95, p’s > .026.
Measures
Child Conduct Problems.
Our outcome of interest was child conduct problems, assessed via the number of child conduct disorder symptoms endorsed by mothers on the Preschool Age Psychiatric Assessment (Egger & Angold, 2006; Egger et al., 2006). The PAPA is a diagnostic interview collected at an in-home assessment which occurred at age 8 for Cohort I and age 6 for Cohort II. The PAPA is based on the Child and Adolescent Psychiatric Assessment for 9–18 year olds, with revisions of content and structure to be more developmentally appropriate for younger children, and excluding six Conduct Disorder items that are age-inappropriate (e.g., truancy, stealing cars, and breaking curfew; see Egger et al., 2006 for details). See Table 1 for sample descriptive statistics. There were no differences in the number of symptoms reported for boys and girls, MBoys = 0.77, MGirls = 0.65, t(393) = 1.28, p = .20. There were no differences in the number of symptoms reported in each cohort, despite the age difference at the time of assessment, MCI = 0.68, MCII = 0.77, t(390) = −0.93, p = .33. Forty-five percent of children presented with at least one symptom; 7% qualified for a conduct disorder diagnosis.
To approximate change in child conduct problems, we also included the most proximal prior measure of child externalizing problems: the Child Behavior Checklist (Achenbach, 2009) at the 4.5 year assessment for Cohort II and the 7 year assessment for Cohort I. The maximum score across AP1 (more often mother) and AP2 (more often father) was used. Both adoptive parents’ scores were internally consistent (α > .89 for across cohorts). The cross-parent association was modest (r = .53, p < .05). The AP1 score was used for 44% families; the AP2 score was used for 30% of the sample; both parents reported identical scores in 26% of the sample. Externalizing problems indicated by T scores of 60 (e.g., at least sub-clinical levels) or greater were present for 25% of the sample; clinical levels (e.g., T-scores of 63 or greater) were indicated for 14% of the sample. By including earlier externalizing problems, we simultaneously change the interpretation of the outcome, conduct problems, to be a measure that encompasses a transition or shift toward conduct problems specifically (e.g., residualizing out common variance between earlier externalizing problems more broadly leaves conduct problem-specific variance to be predicted, and residualizing out earlier externalizing problems that are already relevant to conduct problems allows us to predict residualized change in conduct problems).
Inherited Risk.
BP characteristics are used to index inherited risk. We used principal components analyses (PCA) to create composite indicators of genetic risk for externalizing problems and for internalizing problems (see analytic strategy for score creation).1 Externalizing and internalizing scores were each based on four indicators: (1) number of disorders the BP qualified for on a diagnostic interview (2) number of symptoms the BP endorsed on a diagnostic interview, (3) age of onset of each disorder, (4) proportion of first degree relatives endorsing the same class of problems. Scores built on similar logic (in preliminary form) were used in (BLINDED). Using this approach, we assumed that more symptoms indicate increased severity, and that having symptoms that do not reach the threshold for disorder constitutes a (weaker) genetic influence (e.g., Andrews, Stewart, Allen, & Henderson, 1990; Levy, Hay, McStephen, Wood, & Waldman, 1997). This perspective also is supported by twin studies showing genetic influences on symptom severity in normative populations. We also assumed that crossing from symptoms to disorder increases the genetic loading (Eley, 1997). We assumed that earlier ages of onset are associated with higher genetic risk (supported particularly for depression, (Cadoret, Woolson, & Winokur, 1977; Levinson, 2006; Turner et al., 1993). Finally, we assumed that the number of first degree relatives confers additional genetic risk, or at least is a marker of higher genetic influence (Hettema, Neale, & Kendler, 2001; Sullivan, Neale, & Kendler, 2000).
Three of the indicators used to index genetic risk for externalizing problems were derived from the conduct disorder and antisocial personality disorder of the Diagnostic Interview Schedule (DIS, Robins et al., 1981) and alcohol abuse and dependence, drug abuse and dependence, and tobacco dependence drawn from the Composite International Diagnostic Interview (CIDI; Kessler & Üstün, 2004). Externalizing problems and substance use were combined into a common factor because of the literature showing high levels of genetic overlap in these constructs (e.g., Hicks et al., 2011; Vrieze et al., 2013). Thus, this measure of inherited risk for externalizing problems is designed to capture the maximal inherited risk possible within the confines of the adoption design. The externalizing score included (1) disorders (range = 0 to 7), (2) symptoms (range = 0 to 70), and (3), the youngest age of onset of antisocial and conduct disorder as measured on the DIS, and of alcohol abuse and dependence, drug abuse and dependence, and tobacco dependence, assessed via the CIDI. The fourth indicator was the proportion of first degree relatives endorsing externalizing problems. This indicator was calculated as the maximum proportion of first degree relatives [mother, father, and up to three siblings] the BP rated as having externalizing problems and/or substance use problems on three items, “ever had a hot temper, been in fights frequently, or been involved in stealing regularly”, “ever come into contact with the legal system because of things s/he has done (e.g., been arrested, spent time in jail, had a driver’s license revoked)”, and “ever had problems with drugs or alcohol (e.g., drank too much or used drugs on a regular basis, got mean while drinking”.
The first three indicators of genetic risk for internalizing problems were drawn from the CIDI. The internalizing score included (1) diagnoses (range = 0–11), (2) symptoms (range = 0–48) and (3) the youngest age of onset of major depression, brief recurrent depression, dysthymia, separation anxiety, adult separation anxiety, social phobia, agoraphobia (with and without panic), panic disorder, specific phobia, and generalized anxiety, assessed via the CIDI. The proportion of first degree relatives with internalizing problems was calculated on a single item, “ever been diagnosed with depression or anxiety problems that have been treated or recommended for treatment with medication or counseling”.
The DIS and CIDI were assessed at the 18-month assessment for Cohort I and Cohort II, and assessed again at the 56-month assessment for Cohort I. We opted to use the best score available, so if the diagnosis or symptom was present at either assessment (for Cohort I), then it was scored as present. If the diagnosis or symptom was missing at the 18-month assessment and not present at the 56-month assessment, it was scored as not present. However, if the diagnosis or symptom was not present at the 18-month assessment and missing at the 56-month assessment, the item was scored as missing, as the BP may have developed the symptom between assessments and we cannot be sure that it remains absent. For age of onset, the youngest age of onset was used across waves. Indicators using the best scores vs. the 18-month assessment alone were highly correlated in Cohort I (r’s from .63 - .87).2
The following steps were taken to maximize the separation of prenatal and genetic influences: if the age at onset or recency of a given disorder occurred only during the pregnancy, and not before or after (e.g., the disorder was pregnancy-limited), that diagnosis score was coded as absent exclude prenatal influences from the indicator of genetic influences. The same strategy was used to identify symptoms that appeared only during pregnancy, and these were excluded from the symptom count scores in the genetic risk indicator.
Finally, age of onset is necessarily missing for any BP who did not qualify for diagnosis on any disorder. Thus, we adopted the following strategy: In line with the assumption that earlier age of onset confers higher genetic risk, all missing age of onset values were changed to either (a) one year later than the age of the BP at the time of the (later) assessment, if the BP’s age was older than the oldest reported age of onset, or (b) one year later than the oldest reported age of onset in the sample, if the BP’s age was younger than the oldest reported age of onset. For Cohort I, among disorders assessed at both 18 and 56 months, the younger age of onset was used if they differed across waves.
Substance Use during Pregnancy.
Frequency of maternal SDP and ADP was measured via BM self-report using a pregnancy history calendar (adapted version of the life history calendar; Caspi et al., 1996) assessed at 4 months post-partum. In cases where pregnancy use frequency data were missing (most often because of BMs screening out of further substance use during pregnancy questions), if birth mothers reported no lifetime use, or no pregnancy use, frequency of use during pregnancy was assigned a 0 (none). For SDP, mothers were asked about the average number of cigarettes smoked per day in each trimester. The average number of cigarettes smoked per day across all three trimesters was used as the measure of exposure to smoking during pregnancy. Secondhand smoke exposure was also reported by the mothers (e.g., the average number of cigarettes smoked by a person she was living with who smoked cigarettes). The sum score (prorated for missingness) for these items (r = .42, p < .0001) was used as a measure of total smoke exposure during pregnancy. For ADP, a single item measured frequency of use in cohort I: “What was the average number of glasses (or cans) of alcohol you drank a week during these nine months? (1 drink = 1 glass of wine, 1 12oz. beer, or 1oz. of alcohol).” The same questions were assessed in cohort II, except about each trimester separately. To obtain measures that were comparable across cohorts, the average of the three trimester assessments was taken in Cohort II. Sixty-one percent of mothers reported SDP (43% reported smoking herself, 45% reported passive smoke exposure, and 28% reported both smoking herself and passive smoke exposure), and 23% of mothers reported ADP.3
Postnatal Parent-Child Warmth and Hostility.
Warmth and hostility were assessed using adoptive parents’ self-reports on the Iowa Warmth and Hostility scales (Melby & Conger, 2001) at the wave prior to the assessment of child conduct problems to preserve a consistent measurement approach and the tightest time-lag possible, consistent with studies of parenting and child externalizing problems (Pardini et al., 2008; Shaffer et al., 2013): age 7 for Cohort I, and at age 4.5 for Cohort II. Stability in warmth and hostility was high in Cohort I across the 4.5 and 7 year assessments (warmth r = .68, p < .05; hostility: r = .58, p < .05), and zero-order associations were highly consistent for warmth and hostility with conduct problems in both Cohorts (e.g., Cohort I age 7 parenting with age 8 conduct problems r = −.11, ns, for warmth, r = .22, p < .05 for hostility; Cohort II age 4.5 parenting with age 6 conduct problems r = −.07, ns, for warmth, r = .19, p < .05 for hostility).
The maximum score across AP1 and AP2 was used. Both AP’s scores were internally consistent (α > .76 for warmth and hostility across cohorts). Cross-parent associations were modest at each wave (Cohort I warmth: r = .37, hostility: r = .34; Cohort II warmth: r=.42, hostility: r=.35, p’s < .05). The AP1 score was used between 45% and 54% of the time across measures and cohorts, whereas the AP2 score was used between 22% and 42% of the time across measures and cohorts; both parents reported the same level of warmth or hostility between 9% and 23% of the time across measures and cohorts.
Covariates.
We included child age at the last assessment to control for age variation both within and between cohorts. We also included openness/contact in the adoption and knowledge of the other (birth/adoptive) parent as covariates, as openness of the adoption and contact between birth and adoptive parents could facilitate associations between birth and adoptive parents (see Ge et al., 2008 for more information). Openness of the adoption was measured at the initial assessment via BM and AP report on the extent to which they perceived that the adoption was open on a 7-point scale ranging from1 (very closed) to 7 (very open). AP knowledge about the BPs and BM knowledge about the APs were assessed via five items asking about the extent to which the respondent parent knew about the others’ physical health, mental health, ethnic/cultural background, reasons for adoption, and extended family health history. The standardized mean of BM and AP reports was used at the first assessment (i.e., BM at 5 months and AP at 9 months), when levels of openness and contact were highest in the study.
Analytic strategy
Missing Data: Birth Parent Inclusion.
BFs provide 50% of offspring genes. Coupled with the fact that birth mothers provide both genetic and prenatal influences to offspring, BFs are particularly important informants in the current analysis, which aims to separate and jointly examine genetic and prenatal risk for conduct problems. Although we have a high BF recruitment rate relative to other studies of this nature, this is a difficult population to locate and recruit. Thus, there is a substantial amount of missing data on BFs (ranging from 64–70% based on the particular indicator). We chose to handle missing BF data in the PCA analyses that created genetic risk composites. Specifically, we used the r package missMDA (commands estim_ncpPCA and imputePCA; Josse & Husson, 2016) and FactoMineR (command PCA; Lê, Josse, & Husson, 2008) to impute data within the PCA framework (using a regularized algorithm that avoids overfitting issues when there are high amounts of missing data) to form a completed dataset, and then conducted the PCA on that completed dataset. Thus, in hypothesis testing models, all youth had data on genetic risk.4
Missing Data: Hypothesis Testing.
Hypothesis testing models included a genetic risk score and variables indexing prenatal and postnatal influences, outcomes, and covariates. Because of the longitudinal nature of these data, there was some attrition (see Table 1). Missing data were handled during hypothesis testing using Full Information Maximum Likelihood.
Hypothesis testing.
Hypotheses were tested using structural equation modeling in r with the lavaan package (Rosseel, 2012). We used the se=“bootstrap” command to accommodate the skewed nature of the outcome data. Missing data were accommodated using Full Information Maximum Likelihood (FIML). Conduct problems were regressed on genetic risk (β1), prenatal risk (β2), interaction of genetic and prenatal risk (β3), postnatal warmth (β4), postnatal hostility (β5), the interaction of genetic risk and postnatal warmth (β6) and postnatal hostility (β7), the interaction of prenatal risk and postnatal warmth (β8) and postnatal hostility (β9), and the three-way interactions of genetic and prenatal risk with postnatal warmth (β10) and hostility (β11), adoption process covariates openness (β12) and knowledge (β13), child age (β14), and earlier externalizing problems (β16), We also included the other prenatal drug use frequency as an additional covariate (β15). Finally, we also explicitly modeled the association of genetic risk with prenatal risk (ρ1), as they are both provided by the birth mother. All predictors were mean-centered prior to creating interaction terms and to entering into hypothesis testing models. The four main hypothesis testing models included genetic risk assessed from both birth parents’ externalizing or internalizing scores, and also differed in the specific prenatal risk included (SDP, ADP) to examine whether interactions including prenatal risk were specific to specific substances used during pregnancy.
Sex Differences.
We first fit each model with boys and girls unconstrained – all parameter estimates were freely estimated for boys and girls. Then, we ran a second model constraining all intercepts and parameter estimates to be equal for boys and girls. If this constrained model resulted in a decrement in model fit, then we systematically constrained one path estimate at a time for any effect significant for boys, girls, or both, to formally test for sex differences. Thus, we present findings from the fully unconstrained model. If the constrained model did not result in a decrement of fit, results from the constrained model are presented, with a single estimate reflecting the effect for both boys and girls.
Results
Genetic Risk Composites
The PCA of genetic risk for externalizing problems yielded a single factor explaining 46% of the variance (Eigenvalue = 3.69) with most items loading strongly onto the genetic risk factor score (BM: diagnosis count = .90, symptom count = .93, proportion of first degree relatives = .52, age of onset = −.79; BF: diagnosis count = .88, symptom count = .93, proportion of first degree relatives = .68, age of onset = −.77). The PCA of genetic risk for internalizing problems yielded a single factor explaining 33% of the variance (Eigenvalue = 2.68) with most items loading strongly onto the genetic risk factor score (BM: diagnosis count = .83, symptom count = .83, proportion of first degree relatives = .50, age of onset = −.74; BF: diagnosis count = .88, symptom count = .85, proportion of first degree relatives = .12, age of onset = −.77).
Hypothesis Testing
Zero-order correlations
are presented in Table 2. There were associations among the genetic risk variables. Genetic risk scores were associated with SDP, r = .26 - .36, p < .05, but not with ADP, r = .03-.08, p > .05. ADP was not associated with SDP, r = .05, p > .05. There were no associations of genetic risk or prenatal risk with postnatal warmth or hostility, r = −.02 - .03, p > .05. Postnatal warmth and hostility and genetic risk for internalizing, but not genetic risk for externalizing or prenatal risk, were associated with earlier externalizing problems, r = −.21 - .40, p < .05. SDP, postnatal hostility, and earlier externalizing problems were associated with conduct problems, r = .13 - .37, p < .05.
Table 2.
Descriptive statistics and associations among study variables
| Descriptive Statistics | Correlations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic Risk | Prenatal Risk | Postnatal Parenting | Behavior Problems | ||||||||||
| N | Mean | SD | Ext | Int | SDP | ADP | Warm | Host | Earlier Child Ext | ||||
| Genetic Risk | Externalizing | 552 | 0.00 | 1.64 | 1 | ||||||||
| Internalizing | 552 | 0.00 | 1.92 | 0.35* | 1 | ||||||||
| Prenatal Risk | Smoking | 534 | 3.40 | 5.55 | 0.36* | 0.26* | 1 | ||||||
| Alcohol | 532 | 0.67 | 3.18 | 0.08 | 0.03 | 0.05 | 1 | ||||||
| Postnatal Parenting | Warmth | 408 | 39.45 | 2.74 | −0.01 | 0.03 | 0.04 | 0.01 | 1 | ||||
| Hostility | 408 | 11.72 | 3.01 | 0.01 | 0.02 | 0.07 | −0.02 | −0.30* | 1 | ||||
| Behavior Problems | Earlier Child Ext | 410 | 14.72 | 7.12 | 0.04 | 0.10* | 0.15* | −0.05 | −0.21* | 0.49* | 1 | ||
| Conduct | 393 | 0.71 | 0.99 | −0.02 | −0.08 | 0.13* | 0.01 | −0.09 | 0.21* | 0.37* | |||
Table note. Means and standard deviations (SD) are presented for the un-centered variables here, although mean-centered versions were included in regression models. Ext = externalizing; Int = internalizing; SDP = exposure to smoking during pregnancy; ADP = alcohol use during pregnancy; Warm = warmth; Host = hostility.
p < .05.
Model fitting.
Some parameters were consistent across all models. Across all hypothesis testing models, earlier externalizing problems predicted more conduct problems (see β16, Tables 3 & 4). There were no main effects of postnatal warmth of hostility, or covariates (adoption process: openness and knowledge, and age) on conduct problems in any model. Model-specific results are provided below.
Table 3.
Exposure to Smoking During Pregnancy
| Genetic Risk Score | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Externalizing | Internalizing | ||||||||||||
| Boys | Girls | Boys | Girls | ||||||||||
| β | (SE) | β | (SE) | β | (SE) | β | (SE) | ||||||
| −0.093 | *a | 0.041 | 0.037 | 0.054 | −0.153 | * | 0.038 | −0.059 | 0.049 | ||||
| SDP | β2 | 0.041 | * | 0.017 | 0.015 | 0.016 | 0.042 | *a | 0.015 | 0.008 | 0.012 | ||
| G x SDP | β3 | −0.003 | 0.008 | −0.011 | 0.007 | −0.012 | 0.009 | 0.005 | 0.008 | ||||
| Postnatal warmth | β4 | 0.002 | 0.033 | −0.024 | 0.044 | −0.009 | 0.034 | −0.010 | 0.046 | ||||
| Postnatal hostility | β5 | 0.001 | a | 0.037 | 0.010 | 0.037 | 0.019 | 0.036 | 0.014 | 0.034 | |||
| G x Warmth | β6 | 0.017 | a | 0.019 | −0.028 | 0.041 | 0.039 | * | 0.019 | −0.022 | 0.025 | ||
| G x Hostility | β7 | 0.005 | 0.020 | −0.020 | 0.020 | 0.012 | 0.018 | −0.002 | 0.020 | ||||
| SDP x Warmth | β8 | −0.019 | *a | 0.007 | −0.001 | 0.007 | −0.021 | *a | 0.007 | 0.006 | 0.006 | ||
| SDP x Hostility | β9 | −0.009 | 0.007 | −0.001 | 0.006 | −0.005 | 0.007 | −0.004 | 0.005 | ||||
| G x SDP x Warmth | β10 | −0.005 | a | 0.004 | 0.006 | 0.005 | −0.001 | 0.004 | −3.500 | 0.004 | |||
| G x SDP x Hostility | β11 | 0.006 | * | 0.003 | 0.001 | 0.003 | 0.002 | 0.004 | −0.002 | 0.003 | |||
| Covariates | |||||||||||||
| Adoption Process: Openness | β12 | 0.036 | 0.095 | 0.038 | 0.071 | 0.061 | 0.098 | 0.027 | 0.087 | ||||
| Adoption Process: Knowledge | β13 | −0.073 | 0.114 | 0.103 | 0.114 | −0.073 | 0.112 | 0.085 | 0.145 | ||||
| Age | β14 | −0.023 | 0.070 | −0.017 | 0.064 | −0.110 | 0.066 | −0.006 | 0.059 | ||||
| ADP | β15 | 0.117 | 0.087 | 0.003 | 0.060 | 0.161 | 0.084 | 0.006 | 0.056 | ||||
| Previous EXT | β16 | 0.042 | * | 0.013 | 0.049 | * | 0.015 | 0.044 | * | 0.014 | 0.058 | * | 0.018 |
| rG-SDP | ρ1 | 0.929 | * | 0.179 | 1.392 | * | 0.205 | 0.865 | * | 0.192 | 1.042 | * | 0.253 |
Notes. G = Genetic Risk. rG- SDP = the association of genetic risk and the # of cigarettes smoked per day during pregnancy.
p < .05
denotes significant differences between boys and girls.
Table 4.
Alcohol
| Genetic Risk Score | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Externalizing | Internalizing | ||||||||||||
| Boys | Girls | Boys | Girls | ||||||||||
| β | (SE) | β | (SE) | β | (SE) | β | (SE) | ||||||
| −0.053 | 0.057 | 0.035 | 0.050 | −0.048 | 0.089 | −0.043 | 0.080 | ||||||
| ADP | β2 | 0.122 | 0.127 | 0.033 | 0.106 | 0.164 | 0.167 | 0.028 | 0.098 | ||||
| G x ADP | β3 | 0.060 | 0.078 | −0.018 | 0.053 | 0.139 | 0.123 | 0.002 | 0.106 | ||||
| Postnatal warmth | β4 | 0.027 | 0.037 | 0.005 | 0.033 | 0.022 | 0.042 | −0.015 | 0.037 | ||||
| Postnatal hostility | β5 | 0.012 | 0.039 | 0.015 | 0.043 | −0.010 | 0.051 | 0.008 | 0.043 | ||||
| G x Warmth | β6 | 0.035 | a | 0.024 | −0.013 | 0.026 | 0.035 | a | 0.027 | −0.038 | 0.035 | ||
| G x Hostility | β7 | 0.022 | 0.024 | −0.021 | 0.028 | 0.047 | a | 0.036 | −0.029 | 0.043 | |||
| ADP x Warmth | β8 | 0.052 | 0.050 | −0.008 | 0.040 | 0.015 | 0.055 | −0.014 | 0.032 | ||||
| ADP x Hostility | β9 | 0.023 | 0.051 | 0.009 | 0.051 | −0.015 | 0.070 | 0.009 | 0.046 | ||||
| G x ADP x Warmth | β10 | 0.043 | 0.034 | 0.007 | 0.030 | 0.011 | a | 0.037 | −0.073 | 0.050 | |||
| G x ADP x Hostility | β11 | 0.044 | 0.034 | 0.002 | 0.038 | 0.082 | a | 0.051 | −0.048 | 0.063 | |||
| Covariates | |||||||||||||
| Adoption Process: Openness | β12 | 0.012 | 0.093 | 0.036 | 0.077 | 0.042 | 0.096 | 0.020 | 0.082 | ||||
| Adoption Process: Knowledge | β13 | −0.073 | 0.118 | 0.091 | 0.121 | −0.135 | 0.115 | 0.093 | 0.145 | ||||
| Age | β14 | −0.049 | 0.072 | 0.000 | 0.063 | −0.109 | 0.069 | 0.036 | 0.062 | ||||
| SDP | β15 | 0.018 | 0.015 | −0.004 | 0.013 | 0.022 | 0.015 | 0.004 | 0.011 | ||||
| Previous EXT | β16 | 0.051 | * | 0.012 | 0.052 | * | 0.016 | 0.052 | * | 0.014 | 0.056 | * | 0.019 |
| rG-ADP | ρ1 | 0.127 | 0.068 | 0.135 | 0.122 | 0.085 | 0.066 | −0.018 | 0.065 | ||||
Notes. G = Genetic Risk. rG-ADP = the association of genetic risk and the # of drinks consumed per week during pregnancy.
p < .05
denotes significant differences between boys and girls.
SDP.
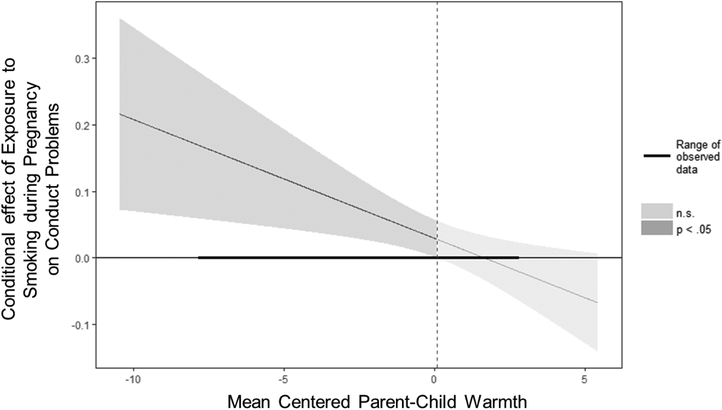
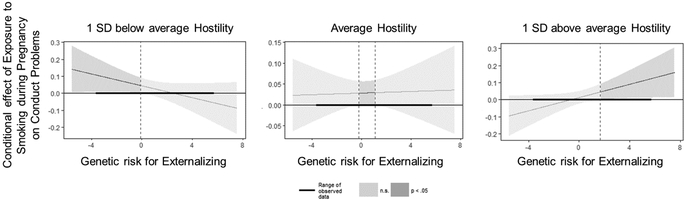
All parameter estimates for models including SDP are presented in Table 3; only significant findings are discussed in text. When examining genetic risk for externalizing problems, constraining all intercepts and parameter estimates to equality across boys and girls did result in a decrement in model fit, χ2change(21) = 47.40, p < .001, full model: χ2(28) = 121.54, AIC = 42,359.22, BIC = 42,540.84; constrained model: χ2(49) = 168.93, AIC = 42,364.61, BIC = 42,455.43. Thus, findings from the full model are presented. There was an association between genetic risk for externalizing problems and SDP for both boys and girls (Table 3, ρ1). This effect did not differ for boys and girls. Genetic risk for externalizing problems predicted fewer conduct problems in boys, but not girls. SDP predicted more conduct problems in boys. Although this effect was not significant for girls, there was not a significant sex difference in the magnitude of effect. These main effects were qualified by interactions of SDP with warmth for conduct problems in boys but not girls. As shown in Figure 1, SDP more strongly predicted higher levels of conduct problems as boys experienced less parental warmth. Further, there was a significant 3-way interaction of genetic risk for externalizing problems, SDP, and hostility, for boys but not girls. As shown in Figure 2, higher exposure to SDP predicted boys’ conduct problems specifically when both genetic risk for externalizing problems and parental hostility were low, when both were average, or when both genetic risk and parental hostility were high. SDP did not predict conduct problems if there was a mismatch in genetic risk and postnatal environments (e.g., at high genetic risk for externalizing but low parental hostility, or low genetic risk for externalizing but high parental hostility).
Figure 1.
Exposure to smoking during pregnancy by parent-child warmth interaction for boys’ conduct problems
Figure 2.
Genetic risk for externalizing problems by exposure to smoking during pregnancy by parent-child hostility interaction for boys’ conduct problems
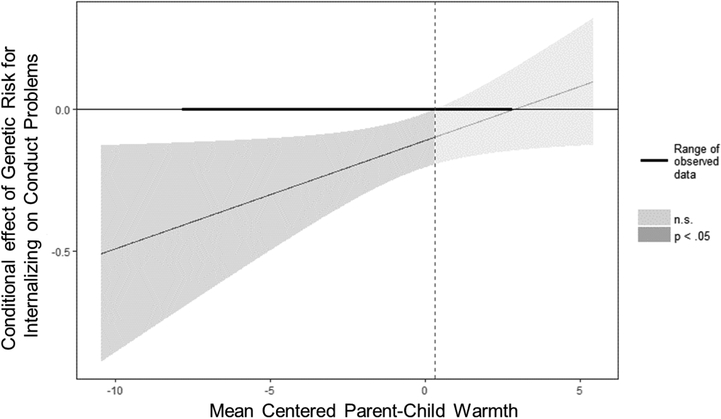
When examining genetic risk for internalizing problems, constraining all intercepts and parameter estimates resulted in a decrement in model fit, χ2 change(21) = 35.46, p = .03, full model: χ2(28) = 96.55, AIC = 41,847.55, BIC = 42,029.17; constrained model: χ2(49) = 132.01, AIC = 41,841.01, BIC = 41,931.82. Thus, findings from the full model are presented. Genetic risk for internalizing problems predicted fewer conduct problems in boys (Table 3, β1). Although this effect was not significant for girls, there was not a significant sex difference in the magnitude of effect. This main effect was qualified by an interaction with warmth for boys (Table 3, β6), but not girls (although the sex difference was not significant). Specifically, the protective effect of genetic risk for internalizing problems on conduct problems was only significant among youth with below-average levels of parent-child warmth (Figure 3). SDP predicted more conduct problems in boys, but not girls (the sex difference was significant). This main effect for boys was qualified by a significant SDP × warmth interaction (Table 3, β8; the effect was not significant for girls, and there was a significant sex difference in magnitude). This is the same interaction presented in the model including genetic risk for externalizing: the positive association between SDP and conduct problems was significant only among youth with below-average levels of parent-child warmth (Figure 1).
Figure 3.
Genetic risk for internalizing by postnatal warmth during pregnancy interaction for boys’ conduct problems
ADP.
Parameter estimates for models including ADP are presented in Table 4. When examining genetic risk for externalizing problems, constraining all intercepts and parameter estimates to equality across boys and girls did result in a decrement in model fit, χ2change(21) = 139.79, p < .001, full model: χ2(28) = 795.33, AIC = 36,558.26, BIC = 36,739.88; constrained model: χ2(49) = 931.12, AIC = 36,652.05, BIC = 36,742.87. Thus, findings from the full model are presented. There were no significant effects in the model aside from the main effect of earlier externalizing problems on conduct problems already noted.
When examining genetic risk for internalizing problems, constraining all intercepts and parameter estimates did result in a decrement in model fit, χ2change(21) = 133.27, p < .001, full model: χ2(28) = 647.81, AIC = 34,236.71, BIC = 35,418.33; constrained model: χ2(49) = 781.08, AIC = 35,327.98, BIC = 35,418.79. Thus, findings from the full model are presented. There were no significant effects in the model aside from the main effect of earlier externalizing problems on conduct problems already noted.
Discussion
We presented novel findings that build on prior theory and literature to fill an important gap in the literature: the relative lack of studies that actually examine genetic, prenatal, and postnatal influences together. We were able to accomplish this complex analytical goal by harnessing the strengths of a large sample of children adopted at birth into non-relative families and their birth and adoptive parents followed longitudinally from 5 months post-partum to, in this study, 6–8 years of age. Another strength of the study is our comprehensive measures of genetic risk for use in adoption designs that are derived from information about symptoms, diagnoses, age of onset, and proportion of first degree relatives with internalizing and externalizing (and available in the online supplement, composite and substance use-specific) problems, including careful consideration of the timing of symptoms and diagnoses in birth mothers to more cleanly separate genetic from prenatal influences.
Main Effects
We found consistent evidence, in line with large bodies of literature, that earlier externalizing problems predicted more conduct problems (Loeber & Burke, 2011), and that SDP predicted more conduct problems (Estabrook et al., 2015). SDP was also associated with genetic risk for internalizing and externalizing problems (which included substance use; Marceau, Hajal, et al., 2013). These results are not surprising; instead they provide replication of expected main effects. Unexpectedly, ADP did not predict more conduct problems and was not associated with genetic risk for externalizing or internalizing problems.
The main effect of parent hostility (found at the zero-order level) disappeared when the effects of earlier externalizing problems were controlled – the effect persisted in models including all interaction terms but not models that included earlier externalizing problems. This somewhat surprising finding is highly suggestive of evocative gene-environment correlation and consistent with bidirectional influences of parental hostility and externalizing problems often found in the literature (e.g., Marceau, Horwitz, et al., 2013; Pardini et al., 2008; Shaffer et al., 2013). That is, in the case of evocative gene-environment correlation, children’s earlier externalizing problems are likely eliciting more hostility from their parents later in development. That hostility mays not actually then increase the risk of youth transitioning from those externalizing behaviors to increased conduct problems– that transition is, in this case, better explained by the earlier level of externalizing behaviors than by the parents’ earlier hostility. However, given that bidirectional influences are found even in genetically informed studies (e.g., Burt, McGue, Krueger, & Iacono, 2005; Neiderhiser, Reiss, Hetherington, & Plomin, 1999), and are only partly influenced by children’s genes, hostile parenting likely also exerts an environmental influence exacerbating the parent hostility-externalizing transactions over time. In addition to the result of evocative gene-environment correlation, it may be that stability in externalizing problems impeded our ability to find statistically significant predictive main effects of parenting on the development of conduct problems in middle childhood in this sample.
Finally, we did not find consistent main effects of genetic risk for externalizing or internalizing problems, although genetic influences were important in interaction with prenatal and parenting influences, particularly for boys. Both high and low genetic risk for externalizing problems was shown to contribute to conduct problems, depending on the prenatal and postnatal contexts. Thus, our findings highlight the importance of considering both the prenatal and postnatal context for understanding how genetic influences guide the development of conduct problems in boys. In contrast to the pattern for genetic risk for externalizing problems, genetic risk for internalizing problems was somewhat surprisingly protective against conduct problems, but only in a specific postnatal context: low parental warmth. Interestingly, some data have shown that children’s, particularly boys’, internalizing problems can reduce the risk of conduct problems, likely through behavioral inhibition (Kerr, Tremblay, Pagani, & Vitaro, 1997). It is possible that in this study, the genetic influences uncovered in boys that are distinct from the genetic risk associated with SDP exposure (as this pattern of findings was found only for boys in models including SDP) are those that drive behavioral inhibition. In that case, then findings that behavioral inhibition and internalizing problems can be protective in boys may be driven by a genetic predisposition to internalizing that is not shared with SDP risk. Although at this point speculative, this interpretation is a testable hypothesis for future researchers to explore.
Hypothesized gene × environment interactions
Our main hypotheses centered on G × E interactions. We generally found evidence of sex differences, particularly in the interactions, highlighting the need for future work on conduct problems to consider sex differences even in childhood. As we anticipated, there were more interactive effects for boys than girls. These findings are in line with prior work suggesting that boys are more vulnerable to early risk factors, (Kraemer, 2000) and that interactive effects of genetic influences with early environmental risks serve as risk for externalizing problems in boys in particular (e.g., Becker et al., 2008; Byrd & Manuck, 2014). These results should be considered preliminary (see limitations below), but nonetheless provide evidence that sex differences are non-ignorable in the development of conduct problems in childhood.
First, we expected that heritable risk for conduct problems would surface in the context of high parental hostility and low parental warmth, but be absent in conditions of average-to-low hostility and average-to-high parental warmth. This two-way interaction was supported for genetic risk for internalizing problems × parental warmth in boys when SDP was included in the model. This interaction provides some support of our first hypothesis, although on the whole, we did not find significant gene × parenting environment interactions. It may be that including SDP in the model explains some of the variance typically attributed to gene × postnatal environment interactions. Possibly, the only gene × parenting environment interaction strong enough to survive controls for earlier externalizing problems, SDP, and ADP is genetic risk for internalizing problems × parental warmth. Warmth is increasingly understood as a particularly salient postnatal influence (Burt et al., 2013; Pardini, Waller, & Hawes, 2015; Reuben et al., 2016), and there was more variability in warmth than hostility in this sample. However, why warmth would work in conjunction with genetic risk for internalizing problems specifically in this sample is somewhat puzzling. This pattern of findings may have emerged because SDP and ADP were more strongly associated with genetic risk for externalizing problems (which includes substance use behaviors), and so more of the genetic risk specific to externalizing problems might be overlapping with the influence of prenatal factors, especially based on evidence of familial confounding in many SDP – externalizing associations (D’Onofrio et al., 2008).
Second, we expected that SDP and ADP would exacerbate heritable risk for conduct problems in children. We found no support for this set of hypotheses outside of the context of a three-way interaction (discussed below). However, in initial analyses designed to vet the genetic risk scores (available from author upon request), we did find some evidence of gene × SDP interactions. These interactions were no longer significant in the context of the full model including covariates and postnatal environmental influences (i.e., earlier externalizing problems and parenting influences in particular). This pattern of findings underscores the importance of examining multiple influences together when complex theoretical models of development are expected. Although potentially present, our findings suggest that gene × prenatal interactions are unlikely to be robust enough to be meaningful targets of intervention in the context of a whole developing child embedded in a family unit – at least for families with lower levels of hostility and conduct problems like this sample. A similar analysis in a higher-risk group of families based on levels of hostility and conduct problems may well show significant gene × prenatal interactions even in a broader context. This work must be done before strong conclusions can be drawn.
Although not explicitly hypothesized because of the relative lack of literature to draw on, we did find some evidence of prenatal × parenting interactions. The positive association between SDP and conduct problems was significant only among boys with below-average levels of parental warmth, not among boys with high parental warmth, in the SDP only analyses. This interaction is in line with the idea that later environmental influences can modify the effect of earlier, ‘set’ influences (Reiss, Leve, & Neiderhiser, 2013). Interactions in this direction lend support to the potential effectiveness of interventions seeking to mitigate the longer-term effects of early-occurring risks that could not be prevented.
Finally, we expected three-way interactions of heritable, prenatal, and parenting influences – particularly that low parental warmth and/or high parental hostility would exacerbate the expected two-way gene × prenatal interactions. In general, we did not find many significant three-way interactions: only one was significant. Whereas we did support that parental hostility moderated the two-way gene x prenatal interaction, it was actually the combination of genetic and postnatal environmental risk that exacerbated or ameliorated the main effect of SDP (as opposed to both environmental contexts exacerbating the main effect of genetic influences, as hypothesized). Specifically, it seemed that “matching” genetic and postnatal environments allowed for the expression of SDP risk, whereas mismatches in genetic and postnatal risk suppressed the effect of SDP. We did see the expected pattern whereby the highest levels of all three influences (high genetic risk, exposure to SDP, and postnatal hostility) predicted conduct problems. However, we also found that the effect of SDP was strong in the absence of genetic and postnatal risk. This finding supports the potential for SDP to exert a meaningful influence on boys’ conduct problems under certain conditions (e.g., when familial confounds are absent, consistent with Gaysina et al., 2013). Because we are the first to observe this interaction, it must be replicated before it is interpreted meaningfully.
Limitations
When interpreting these results, several limitations should be considered. The first important limitation is our inability to perfectly separate genetic from prenatal influences. Our design and measurement strategy improve on existing methods and provide one of the best tests available. However, despite our best efforts to separate genetic from prenatal influences, the fact remains that birth mothers provide reports of both influences. Maternal reports of both influences increase the likelihood of the genetic-prenatal correlations reported were inflated, at least in part due to shared rater effects.
One way we attempted to mitigate this bias was by including BF information when available. Sensitivity analyses with BFs alone showed genetic-prenatal correlations for tobacco and drug use, although the interactions were driven by birth mother genetic risk, so while some findings may have been influenced by rater bias, there are also likely some ‘real’ effects. Importantly, the sensitivity analyses were extremely limited by missing data. Indeed, the majority of BF data were missing for the genetic risk scores presented here. There is evidence that these data can be imputed successfully (Blozis et al., 2013), and we believe that the inclusion of the data in this study, despite high amounts of missingness, is important theoretically to best capture heritable risk and to help disentangle heritable from prenatal risk. We are further convinced that the effects are not overly biased based on the pattern of findings being very similar to that found when only birth mother data were used to estimate heritable risk.
Despite our large sample size, especially for such a specialized design, we may be underpowered to detect small interactive effects. Although our a priori power analyses supported that we are likely to have sufficient power to detect the two-way interaction effects, we were likely underpowered to detect the small three-way interactions found, and thus the three-way interaction effects should be viewed as preliminary. It is also important to note that our sample is not a clinical sample. Rates of externalizing problems and parental hostility are relatively low, which also limits our power to find significant effects that are more likely to be small given the variability in the sample. The goal of this paper was not to provide definitive answers, but rather 1) to highlight the need for testing the complex theories of the development of conduct problems more fully by including multiple focal influences together, and 2) provide preliminary findings that others can now replicate (or not) that inform theory for which influences are more or less important in conjunction with others. We also had few interaction effects relative to the total number of tests - they do not survive correction for multiple testing and thus are in need of replication, although it is important to note that replication of these findings may not be realistic due to the unique nature of our study design. As such, we have taken care to interpret the findings as preliminary at applying to normal development and normative levels of externalizing problems, and highly encourage readers to do the same.
We approximate change in conduct problems using earlier externalizing problems, however, the earlier and later measures of externalizing problems are not the same. That is, conduct problems were assessed via a symptom count from a diagnostic interview assessing specifically conduct disorder, whereas earlier externalizing problems were assessed via a broad checklist measure tapping several aspects of externalizing problems (aggression vs. rule breaking; attention, oppositional, and conduct problems). Thus, interactions (which were only present after controlling for earlier externalizing problems) could be specific to change in or the transition to conduct problems, or findings could be specific to conduct problems that are not overlapping with other externalizing problems. Based on sensitivity analyses including and excluding earlier externalizing problems and using conduct disorder specifically vs. broader indexes of disruptive behavior, it appears that the best interpretation is some of each. That is, the interactions observed here are replicated using other measurement and model building strategies, but are strongest as presented, when the outcome measure reflects a transition from broader externalizing problems to conduct problems specifically. Coupled with the body of work suggesting that early externalizing problems lead into conduct problems, and that there are trajectories of conduct problem development that cross specific externalizing disorders (Loeber & Burke, 2011), we have chosen to interpret our findings as predicting the transition to conduct problems. However, future work using the same measure repeated over time would help to clarify these effects. Other measurement limitations include the reliance of only parent reports of their parenting, and retrospective measurement of SDP and ADP. It would be interesting and important to determine whether results replicate when using, for example, observed parenting behaviors and prospectively collected and/or objectively verified prenatal exposures. Finally, the inclusion of earlier externalizing problems as a covariate may mask or bias the magnitude of prenatal influences on conduct problems, insofar as these effects are associated with earlier externalizing problems and are ‘partialled out’ in the full regression analyses.
Conclusions
This paper presented preliminary findings that highlight three main points. First, SDP played an important role for the development of boys’ conduct problems, even above comorbid ADP and controlling on genetic and postnatal influences and interactions. Second, some well-accepted effects (e.g., the effect of parental hostility; gene × SDP interactions) were not as important when considering a fuller picture of the development of conduct problems. Finally, main effects and interactions among genetic, prenatal, and postnatal (parenting) influences on conduct problems appeared specific to boys, suggesting that sex differences are particularly important to consider in future work. Our study design has many strengths, including the genetically-informed design, use of data on birth fathers, and developmental conceptualization and analysis. Our findings thus provide empirically-grounded hypotheses for future research that can circumvent some of our study limitations to further test complex developmental models.
Supplementary Material
Acknowledgements.
This project was supported by grant R01 MH092118 from the National Institute of Mental Health, NIH, U.S. PHS. This project was also supported by grants R01 DA020585 and R01 DA045108 from the National Institute on Drug Abuse, the National Institute of Mental Health and OBSSR, NIH, U.S. PHS and R01 HD042608 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the National Institute on Drug Abuse, NIH, U.S. PHS, and UG3 OD023389 from the Office of the Director. Dr. Marceau was supported by the National Institute on Drug Abuse K01 DA039288. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Separate externalizing, internalizing, and substance use indicators, as well as a general psychopathology (p-score) has also been developed for this study and are presented in the technical report in supplemental materials. Results using separated externalizing and substance use genetic indicators produced highly similar results, and thus given evidence that substance use and externalizing problems have highly overlapping heritability (Hicks, Schalet, Malone, Iacono, & McGue, 2011), we elected to use the larger externalizing factor described here to index genetic influences in the current paper and reduce the quantity of models and findings presented. These additional analyses are available upon author request.
Sensitivity analyses using factor scores based on indicators from the 18-month assessment vs. the ‘best’ assessment were highly consistent (available in the technical report, supplemental materials).
Because some mothers reported SDP and ADP (n = 83; 15% of the sample), we also created a composite indictor that was the SDP/ADP composite frequencies (after they had been standardized to M = 0, SD = 1 to make the scaling of the frequency values comparable). We repeated hypothesis testing models with this variable, but interaction findings were not significant, indicating that findings are substance-specific. Analyses available upon author request.
We also conducted factor scores via PCA using list-wise deletion (complete cases only), and extensively tested the variations on the score, including associations between the listwise-deleted and imputed scores, and the predictive utility of each type of score. Results from these analyses are available in the technical report (supplemental materials). Generally, patterns of findings were very similar across scores.
References
- Achenbach TM (2009). Achenbach system of empirically based assessment (ASEBA): Development, findings, theory, and applications: University of Vermont, Research Center of Children, Youth & Families. [Google Scholar]
- Andrews G, Stewart G, Allen R, & Henderson AS (1990). The genetics of six neurotic disorders: a twin study. Journal of Affective Disorders, 19(1), 23–29. [DOI] [PubMed] [Google Scholar]
- Becker K, El-Faddagh M, Schmidt MH, Esser G, & Laucht M (2008). Interaction of dopamine transporter genotype with prenatal smoke exposure on ADHD symptoms. The Journal of Pediatrics, 152(2), 263–269. e261. [DOI] [PubMed] [Google Scholar]
- Blozis SA, Ge X, Xu S, Natsuaki MN, Shaw DS, Neiderhiser J, … Reiss D (2013). Sensitivity analysis of multiple informant models when data are not missing at random. Structuural Equation Modeling, 20(2), 283–298. doi: 10.1080/10705511.2013.769393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan LM, & Shaw DS (2013). Revisiting data related to the age of onset and developmental course of female conduct problems. Clinical Child and Family Psychology Review, 16(1), 35–58. doi: 10.1007/s10567-012-0125-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Hammen C, Sylvers P, Bor W, Najman J, Lind P, … Smith AK (2011). Interactions between the COMT Val108/158Met polymorphism and maternal prenatal smoking predict aggressive behavior outcomes. Biological Psychology, 87(1), 99–105. doi: 10.1016/j.biopsycho.2011.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublitz MH, & Stroud LR (2012). Maternal smoking during pregnancy and offspring brain structure and function: review and agenda for future research. Nicotine & Tobacco Research, 14(4), 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT, & Kerbeshian J (2003). Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicology and Teratology, 25(6), 697–705. [DOI] [PubMed] [Google Scholar]
- Burt SA (2009). Are there meaningful etiological differences within antisocial behavior? Results of a meta-analysis. Clinical Psychology Review, 29, 163–178. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klahr AM, Neale MC, & Klump KL (2013). Maternal warmth and directiveness jointly moderate the etiology of childhood conduct problems. Journal of Child Psychology and Psychiatry, 54(10), 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, & Klump K (2013). Delinquent peer affiliation as an etiological moderator of childhood delinquency. Psychol Med, 43(06), 1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, & Klump K (2014). Parent–child conflict as an etiological moderator of childhood conduct problems: an example of a ‘bioecological’gene–environment interaction. Psychological Medicine, 44(05), 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klump KL, Gorman-Smith D, & Neiderhiser JM (2016). Neighborhood disadvantage alters the origins of children’s nonaggressive conduct problems. Clinical Psychological Science, 4(3), 511–526. doi: 10.1177/2167702615618164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, & Iacono W (2003). Parent-child conflict and the comorbidity among childhood externalizing disorders. Archives of General Psychiatry, 60(5), 505–513. [DOI] [PubMed] [Google Scholar]
- Burt SA, McGue M, Krueger RF, & Iacono WG (2005). How are parent-child conflict and childhood externalizing symptoms related over time? Results from a genetically informative cross-lagged study. Development & Psychopathology, 17, 145–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TMM, Lau JYF, Maughan B, & Eley TC (2008). Parental punitive discipline, negative life events and gene-environment interplay in the development of externalizing behavior. Psychological Medicine, 38(1), 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, & Manuck SB (2014). MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biological Psychiatry, 75(1), 9–17. doi: 10.1016/j.biopsych.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret R, Cain CA, & Crowe RR (1983). Evidence for gene-environment interaction in the development of adolescent antisocial behavior. Behavior Genetics, 13(3), 301–310. [DOI] [PubMed] [Google Scholar]
- Cadoret R, Woolson R, & Winokur G (1977). The relationship of age of onset in unipolar affective disorder to risk of alcoholism and depression in parents. Journal of Psychiatric Research, 13(3), 137–142. doi: 10.1016/0022-3956(77)90002-4 [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Thornton A, Freedman D, Arnell JW, Harrington H, … Silva PA (1996). The life history calendar: A research and clinical assessment method for collecting retrospective event-history data. International Journal of Methods in Psychiatric Research, 6(2), 101–114. [Google Scholar]
- D’Onofrio BM, Slutske WS, Turkheimer E, Emery RE, Harden KP, Heath AC, … Martin NG (2007). Intergenerational transmission of childhood conduct problems: A children of twins study. Archives of General Psychiatry, 64(7), 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer EN, Eaves LJ, Corey LA, Berg K, Solaas MH, & Emery RE (2003). The role of the Children of Twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry, 44(8), 1130–1144. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, & Lahey BB (2008). Smoking during pregnancy and offspring externalizing problems: An exploration of genetic and environmental confounds. Development & Psychopathology, 20(1), 139–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA (2009). Mechanisms of gene-environment interaction in the development of conduct disorder. Perspectives on Psychological Science, 4(4), 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, & Angold A (2006). Common emotional and behavioral disorders in preschool children: Presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry, 47(3/4), 313–337. [DOI] [PubMed] [Google Scholar]
- Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, & Angold A (2006). Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA). Journal of the American Academy of Child & Adolescent Psychiatry, 45(5), 538–549. [DOI] [PubMed] [Google Scholar]
- Ekblad M, Korkeila J, & Lehtonen L (2015). Smoking during pregnancy affects foetal brain development. Acta paediatrica, 104(1), 12–18. [DOI] [PubMed] [Google Scholar]
- Eley TC (1997). Depressive symptoms in children and adolescents: Etiological links between normality and abnormality: A research note. Journal of Child Psychology & Psychiatry & Allied Disciplines, 38(7), 861–865. [DOI] [PubMed] [Google Scholar]
- Ellingson JM, Goodnight J, Van Hulle C, Waldman I, & D’Onofrio B (2014). A sibling-comparison study of smoking during pregnancy and childhood psychological traits. Behavior Genetics, 44(1), 25–35. doi: 10.1007/s10519-013-9618-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson JM, Rickert ME, Lichtenstein P, Langstrom N, & D’Onofrio BM (2012). Disentangling the relationships between maternal smoking during pregnancy and co-occurring risk factors. Psychological Medicine, 42(7), 1547–1557. doi: 10.1017/s0033291711002534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, & Robinson ML (2001). Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child & Adolescent Psychiatry, 40(6), 630–641. [DOI] [PubMed] [Google Scholar]
- Estabrook R, Massey SH, Clark CA, Burns JL, Mustanski BS, Cook EH, … Wakschlag LS (2015). Separating family-level and direct exposure effects of smoking during pregnancy on offspring externalizing symptoms: Bridging the behavior genetic and behavior teratologic divide. Behavior Genetics. doi: 10.1007/s10519-015-9762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg ME, Button TMM, Neiderhiser JM, Reiss D, & Hetherington EM (2007). Parenting and adolescent antisocial behavior and depression: Evidence of genotype x parenting environment interaction. Archives of General Psychiatry, 64(4), 457–465. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, & Mattson SN (2007). Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics, 119(3), e733–e741. [DOI] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, … Harold GT (2013). Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry, 70(9), 956–963. doi: 10.1001/jamapsychiatry.2013.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Cadoret RJ, Neiderhiser JM, Yates W, & Troughton E (1996). The developmental interface between nature and nurture: A mutual influence model of child antisocial behavior and parent behaviors. Developmental Psychology, 32(4), 574–589. [Google Scholar]
- Ge X, Natsuaki MN, Martin DM, Leve LD, Neiderhiser JM, Shaw DS, … Reiss D (2008). Bridging the divide: Openness in adoption and postadoption psychosocial adjustment among birth and adoptive parents Journal of Family Psychology, 22(4), 529–540. doi: 10.1037/a0012817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V (2011). Annual research review: prenatal stress and the origins of psychopathology: an evolutionary perspective. Journal of Child Psychology and Psychiatry, 52(4), 356–367. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, & Kendler KS (2001). A review and meta-analysis of the genetic epidemiology of anxiety disorders. American Journal of Psychiatry, 158(10), 1568–1578. doi: 10.1176/appi.ajp.158.10.1568 [DOI] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, & McGue M (2011). Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior Genetics, 41(4), 459–475. doi: 10.1007/s10519-010-9417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S, Zohsel K, Buchmann AF, Blomeyer D, Holz N, Boecker-Schlier R, … Schmidt MH (2016). Interacting effect of MAOA genotype and maternal prenatal smoking on aggressive behavior in young adulthood. Journal of Neural Transmission, 123(8), 885–894. [DOI] [PubMed] [Google Scholar]
- Holz NE, Zohsel K, Laucht M, Banaschewski T, Hohmann S, & Brandeis D (2016). Gene x environment interactions in conduct disorder: Implications for future treatments. Neuroscience & Biobehavioral Reviews. [ePub ahead of print] [DOI] [PubMed] [Google Scholar]
- Josse J, & Husson F (2016). missMDA: A package for handling missing values in multivariate data analysis. Journal of Statistical Software, 70(1), 31. doi: 10.18637/jss.v070.i01 [DOI] [Google Scholar]
- Kahn RS, Khoury J, Nichols WC, & Lanphear BP (2003). Role of dopamine transporter genotype and maternal prenatal smoking in childhood hyperactive-impulsive, inattentive, and oppositional behaviors. The Journal of Pediatrics, 143(1), 104–110. [DOI] [PubMed] [Google Scholar]
- Kerr M, Tremblay r. E., Pagani L, & Vitaro F (1997). Boy’s behavioral inhibition and the risk of later delinquncy. Archives of General Psychiatry, 54, 809–816. [DOI] [PubMed] [Google Scholar]
- Kessler RC, & Üstün TB (2004). The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13(2), 93–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS (2009). Maternal smoking during pregnancy and child outcomes: Real or spurious effect? Developmental Neuropsychology, 34(1), 462593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, … Martin NG (2006). Maternal alcohol use disorder and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine, 36(10), 1461–1471. [DOI] [PubMed] [Google Scholar]
- Kraemer S (2000). The fragile male. BMJ : British Medical Journal, 321(7276), 1609–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuja-Halkola R, D’Onofrio BM, Larsson H, & Lichtenstein P (2014). Maternal Smoking During Pregnancy and Adverse Outcomes in Offspring: Genetic and Environmental Sources of Covariance. Behavior Genetics, 44(5), 456–467. doi: 10.1007/s10519-014-9668-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Holmans PA, van den Bree MB, & Thapar A (2007). Effects of low birth weight, maternal smoking in pregnancy and social class on the phenotypic manifestation of Attention Deficit Hyperactivity Disorder and associated antisocial behaviour: investigation in a clinical sample. BMC psychiatry, 7(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê S, Josse J, & Husson F (2008). FactoMineR: An R Package for Multivariate Analysis. Journal of Statsitical Software, 25(1), 18. doi: 10.18637/jss.v025.i01 [DOI] [Google Scholar]
- Leve LD, Kerr DC, Shaw D, Ge X, Neiderhiser JM, Scaramella LV, … Reiss D (2010). Infant pathways to externalizing behavior: Evidence of genotype x environment interaction. Child Development, 81(1), 326–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Neiderhiser JM, Shaw DS, Ganiban J, Natsuaki MN, & Reiss D (2013). The Early Growth and Development Study: A prospective adoption study from birth through middle childhood. Twin Research and Human Genetics, 16(1), 412–423. doi: 10.1017/thg.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF (2006). The genetics of depression: A review. Biological Psychiatry, 60(2), 84–92. doi: 10.1016/j.biopsych.2005.08.024 [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, & Waldman I (1997). Attention-Deficit Hyperactivity Disorder: A category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child & Adolescent Psychiatry, 36(6), 737–744. doi: 10.1097/00004583-199706000-00009 [DOI] [PubMed] [Google Scholar]
- Lipscomb ST, Laurent H, Neiderhiser JM, Shaw DS, Natsuaki MN, Reiss D, & Leve LD (2014). Genetic vulnerability interacts with parenting and early care education to predict increasing externalizing behavior. International Journal of Behavioral Development, 38(1), 70–80. doi: 10.1177/0165025413508708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, & Burke JD (2011). Developmental pathways in juvenile externalizing and internalizing problems. Journal of Research on Adolescence, 21(1), 34–46. doi: 10.1111/j.1532-7795.2010.00713.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkari S, Hakko H, Herva A, Pouta A, Riala K, & Räsänen P (2012). Exposure to obstetric complications in relation to subsequent psychiatric disorders of adolescent inpatients: specific focus on gender differences. Psychopathology, 45(5), 317–326. [DOI] [PubMed] [Google Scholar]
- Marceau K, Hajal N, Leve LD, Reiss D, Shaw DS, Ganiban JM, … Neiderhiser JM (2013). Measurement and associations of pregnancy risk factors with genetic influences, postnatal environmental influences, and toddler behavior. International Journal of Behavioral Development, 37(4), 366–375. doi: 10.1177/0165025413489378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Horwitz BN, Narusyte J, Ganiban JM, Spotts EL, Reiss D, & Neiderhiser JM (2013). Gene-environment correlation underlying the association between parental negativity and adolescent externalizing problems. Child Development, 84(6), 2031–2046. doi: 10.1111/cdev.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Laurent HK, Neiderhiser JM, Reiss D, Shaw DS, Natsuaki MN, … Leve LD (2015). Combined influences of genes, prenatal environment, cortisol, and parenting on the development of children’s internalizing versus externalizing problems. Behavior Genetics, 45(3), 268–282. doi: 10.1007/s10519-014-9689-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Palmer RH, Neiderhiser JM, Smith TF, McGeary JE, & Knopik VS (2016). Passive rGE or developmental gene-environment cascade? An investigation of the role of xenobiotic metabolism genes in the association between smoke exposure during pregnancy and child birth weight. Behavior Genetics, 46(3), 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, & Moffit TE (2004). Prenatal smoking and early childhood conduct problems: Testing genetic and environmental explanations of the association. Archives of General Psychiatry, 61, 836–843. [DOI] [PubMed] [Google Scholar]
- Melby JN, & Conger RD (2001). The Iowa Family Interaction Rating Scales: Instrument summary. [Google Scholar]
- Molnar DS, Levitt A, Eiden RD, & Schuetze P (2014). Prenatal cocaine exposure and trajectories of externalizing behavior problems in early childhood: Examining the role of maternal negative affect. Development & Psychopathology, 26(02), 515–528. doi: 10.1017/S0954579414000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiderhiser JM, Reiss D, Hetherington E, & Plomin R (1999). Relationships between parenting and adolescent adjustment over time: Genetic and environmental contributions. Developmental Psychology, 35(3), 680–692. [DOI] [PubMed] [Google Scholar]
- Neiderhiser JM, Marceau K, De Araujo-Greecher M, Ganiban JM, Mayes LC, Shaw DS Reiss D, & Leve LD (2016). Estimating the roles of genetic risk, perinatal risk, and marital hostility on early childhood adjustment: Medical records and self-reports. Behavior Genetics, 46(3), 334–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, & Breslau N (2007). Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 46(3), 362–369. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, & Glover V (2002). Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. British Journal of Psychiatry, 180, 502–508. [DOI] [PubMed] [Google Scholar]
- Paradis AD, Shenassa ED, Papandonatos GD, Rogers ML, & Buka SL (2017). Maternal smoking during pregnancy and offspring antisocial behaviour: findings from a longitudinal investigation of discordant siblings. Journal of Epidemiology and Community Health. doi: 10.1136/jech-2016-208511 [DOI] [PubMed] [Google Scholar]
- Pardini DA, Fite PJ, & Burke JD (2008). Bidirectional associations between parenting practices and conduct problems in boys from childhood to adolescence: The moderating effect of age and African-American ethnicity. Journal of Abnormal Child Psychology, 36(5), 647–662. doi: 10.1007/s10802-007-9162-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Waller R, & Hawes SW (2015). 13 Familial influences on the development of serious conduct problems and delinquency. The development of criminal and antisocial behavior (pp. 201–220): Springer. [Google Scholar]
- Patterson G, Reid J, & Dishion T (1998). Jenkins Antisocial boys, Jennifer M (Ed); Oatley Keith (Ed); et al. (1998) Human emotions: A reader (pp. 330–336). Malden, MA: ; Malden, MA: Blackwell Publishers Inc.; Blackwell Publishers Inc. [Google Scholar]
- Reiss D, Leve LD, & Neiderhiser JM (2013). How genes and the social environment moderate each other. American Journal of Public Health, 103(S1), S111–S121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben JD, Shaw DS, Neiderhiser JM, Natsuaki MN, Reiss D, & Leve LD (2016). Warm parenting and effortful control in toddlerhood: Independent and interactive predictors of school-age externalizing behavior. Journal of Abnormal Child Psychology, 44(6), 1083–1096. doi: 10.1007/s10802-015-0096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, Hay DF, van den Bree M, & Thapar A (2009). Disentangling prenatal and inherited influences in humans with an experimental design. Proceedings of the National Academy of Sciences, 106(7), 2464–2467. doi: 10.1073/pnas.0808798106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y (2012). lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software, 48(2), 36. doi: 10.18637/jss.v048.i02 [DOI] [Google Scholar]
- Salatino-Oliveira A, Murray J, Kieling C, Genro JP, Polanczyk G, Anselmi L, … Rohde LA (2016). COMT and prenatal maternal smoking in associations with conduct problems and crime: the Pelotas 1993 birth cohort study. Scientific reports, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore JE, & Dick DM (2016). Genetic influences on conduct disorder. Neuroscience & Biobehavioral Reviews. [ePub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer A, Lindhiem O, Kolko DJ, & Trentacosta CJ (2013). Bidirectional effects between parenting practices and child externalizing behavior: A cross-lagged panel analysis in the context of a psychosocial treatment and 3-year follow-up. Journal of Abnormal Child Psychology, 41(2), 199–210. doi: 10.1007/s10802-012-9670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MJ, & Hofer SM (2005). Social context in gene–environment interactions: Retrospect and prospect. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 60(Special Issue 1), 65–76. [DOI] [PubMed] [Google Scholar]
- Shaw DS, & Bell R (1993). Developmental theories of parental contributors to antisocial behavior. Journal of Abnormal Child Psychology, 21(5), 493–518. doi: 10.1007/bf00916316 [DOI] [PubMed] [Google Scholar]
- Shaw DS, Keenan K, & Vondra JI (1994). Developmental precursors of externalizing behavior: Ages 1 to 3. Developmental Psychology, 30(3), 355–364. doi: 10.1037/0012-1649.30.3.355 [DOI] [Google Scholar]
- Shelleby EC, & Shaw DS (2014). Outcomes of Parenting Interventions for Child Conduct Problems: A Review of Differential Effectiveness. Child Psychiatry and Human Development, 45(5), 628–645. doi: 10.1007/s10578-013-0431-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W Jr, Xu ZA, Levin ED, & Slotkin TA (2005). Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction—developmental neurotoxicity of nicotine. Critical Reviews in Toxicology, 35(8–9), 703–711. [DOI] [PubMed] [Google Scholar]
- Slotkin TA (1992). Prenatal Exposure to Nicotine: What Can. Maternal substance abuse and the developing nervous system, 97. [Google Scholar]
- Steinhausen HC, & Spohr HL (1998). Long‐term outcome of children with fetal alcohol syndrome: Psychopathology, behavior, and intelligence. Alcoholism: Clinical and Experimental Research, 22(2), 334–338. [DOI] [PubMed] [Google Scholar]
- Stice E, & Barrera M (1995). A longitudinal examination of the reciprocal relations between perceived parenting and adolescents’ substance use and externalizing behaviors. Developmental Psychology, 31(2), 322–334. doi: 10.1037/0012-1649.31.2.322 [DOI] [Google Scholar]
- Sullivan PF, Neale MC, & Kendler KS (2000). Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry, 157(10), 1552–1562. doi: 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- Turner WM, Cutter HS, Worobec TG, O’Farrell TJ, Bayog RD, & Tsuang MT (1993). Family history models of alcoholism: age of onset, consequences and dependence. Journal of Studies on Alcohol, 54(2), 164–171. [DOI] [PubMed] [Google Scholar]
- Van Lier PA, Der Ende J. v., Koot HM, & Verhulst FC (2007). Which better predicts conduct problems? The relationship of trajectories of conduct problems with ODD and ADHD symptoms from childhood into adolescence. Journal of Child Psychology and Psychiatry, 48(6), 601–608. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, & Hans SL (2002). Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Development & Psychopathology, 14(2), 351–369. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, … Cook EH Jr (2009). Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Moleculary Psychiatry, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E Jr., Benowitz NL, & Leventhal BL (2002). Maternal smoking during pregnancy and severe antisocial behavior in offspring: A review. American Journal of Public Health, 92(6), 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware AL, O’Brien JW, Crocker N, Deweese BN, Roesch SC, Coles CD, … Sowell ER (2013). The effects of prenatal alcohol exposure and attention‐deficit/hyperactivity disorder on psychopathology and behavior. Alcoholism: Clinical and Experimental Research, 37(3), 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M (2007). Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res, 32(10), 1730–1740. doi: 10.1007/s11064-007-9339-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.