Figure 5.
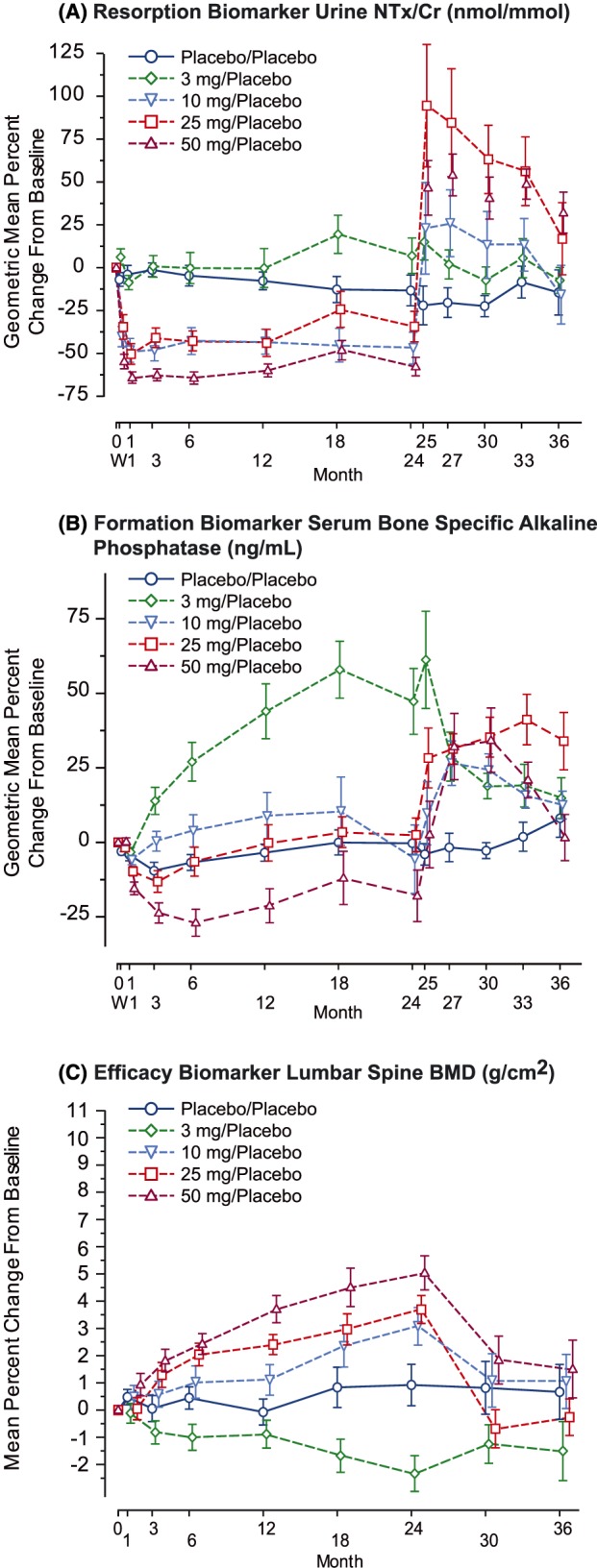
Longitudinal dose–response of key biomarkers over 3 years in the subset of phase 2 (P004) patients switched to placebo at 24 months (geometric mean ± standard error) per‐protocol population placebo.24, 26 A, Resorption biomarker urine aminoterminal crosslinked telopeptides of type 1 collagen to creatinine ratio (NTx/Cr; nmol/mmol). B, Formation biomarker serum bone specific alkaline phosphatase (ng/mL). C, Efficacy biomarker lumbar spine bone mineral density (BMD; g/cm2)
