Figure 1.
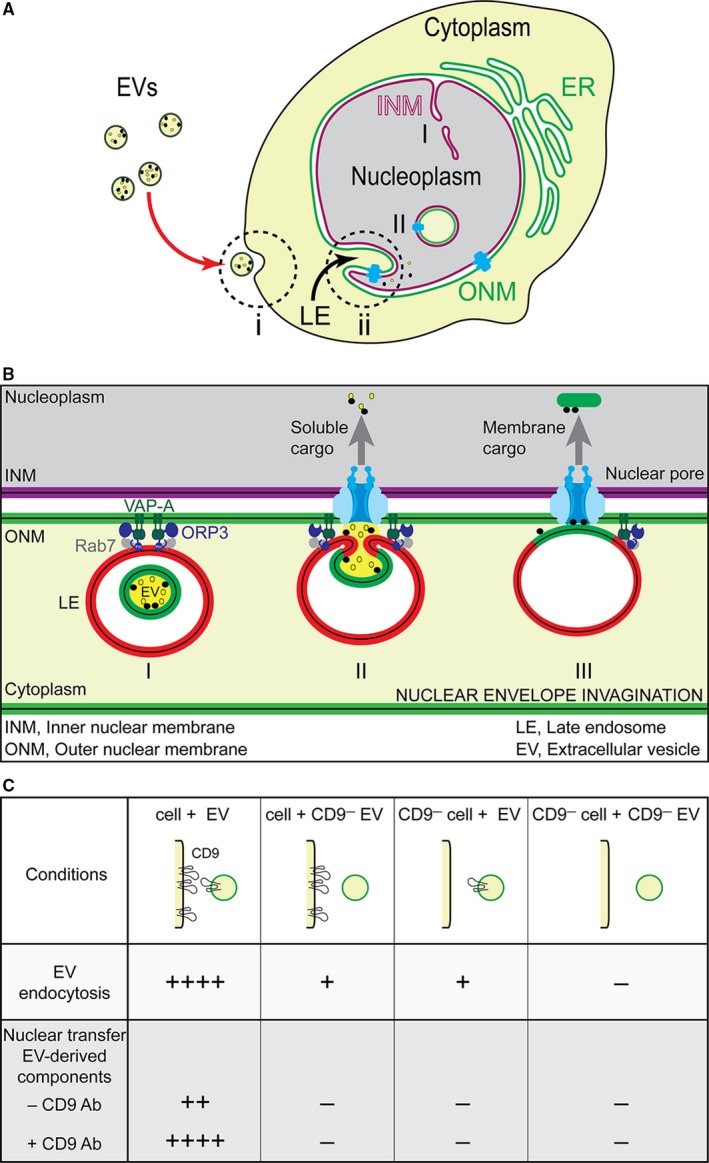
Entry and delivery of extracellular vesicles (EV)‐derived cargo molecules into the nucleoplasm of recipient cells. A, Two major steps were proposed to explain the delivery of EV‐associated molecules to the nuclear compartment of recipient cells. First, the EVs are internalized by endocytosis at the plasma membrane (i). Second, once inside the endocytic pathway, a fraction of late endosomes (LE) penetrates the type II nuclear envelope invaginations where their content, notably the endocytosed EV‐associated molecules, are transferred into the nucleoplasm (ii). Two types of nuclear envelope invaginations are described. Type I invaginations (I) are those in which solely the inner nuclear membrane (INM) penetrates into the nucleoplasm, whereas type II invaginations (II) involve both the outer nuclear membrane (ONM) and INM. The endoplasmic reticulum (ER) is a continuation of ONM. B, Key players involved in the translocation of Rab7+ late endosomes to nuclear envelope invagination. Two proteins, vesicle‐associated membrane protein‐associated protein A (VAP‐A) and the cytoplasmic oxysterol‐binding protein‐related protein 3 (ORP3) forming a tripartite complex with late endosome‐associated Rab7 protein, are indispensable for the entry of late endosomes to the nuclear envelope invagination and/or their tether to ONM (I). Nuclear pores are somehow involved in the translocation of EV‐associated soluble (II) and membranous (III) cargo molecules into the nucleus. It remains to be explained how membranous components of EVs are extracted from the late endosomal membrane upon fusion of the former with the latter and the transport mechanism through nuclear pores, which are size restricted. C, Silencing CD9 in recipient cells and/or EVs or both interferes with the endocytosis of EVs and the nuclear transfer of their cargo molecules. Although the presence of divalent CD9 Ab stimulated these events with native cells and EVs, the lack of CD9 abrogated them.23 Panels A and B were modified from Ref.29
